Rhinology
Vol. 45: Issue 2 - April 2025
Clinicopathological and microbiological study of fungal rhinosinusitis treated with endoscopic surgery
Abstract
Objective. The objective of this study was to analyse the aetiology, clinical presentations, histopathology and microbiological aspects of fungal rhinosinusitis (FRS) in patients undergoing endoscopic surgery.
Methods. The descriptive study was carried out over a 4-year period in two Serbian ENT Clinics and included patients with sinonasal pathology who underwent endoscopic surgery.
Results. The study included 26 patients. The most common forms of FRS treated by endoscopic sinus surgery was allergic FRS (AFRS). The fungus identification rate varied between entities, and was 72.2% in AFRS and 33.3% in fungal ball specimens. The common species seen in AFRS isolates were Cladosporium spp. (38.5% of isolated) and dematiaceous molds in the same percentage, while the remainder of the cultures were hyaline moulds. CT scan can be very helpful in diagnosing FRS and sometimes even in differentiating between different entities. Treatment of FRS should be tailored for each entity. Postoperative medical treatment in AFRS should consider potential advancements described in the literature.
Conclusions. This study emphasises the need to combine all types of clinical, radiology, pathohistological and microbiological methods to obtain the best diagnostic and treatment strategies and should be the basis for further research.
Introduction
Rhinosinusitis is a common disorder with great personal and economic impact. The increased prevalence of chronic rhinosinusitis (CRS) is being seen in recent decades throughout the world, and is estimated to affect 10-20% of the global population depending on the region 1. Increased awareness of the consequences caused by CRS, such as a higher risk of cardiovascular disease and negative effect on quality of life (QOL), have led to many new studies, treatment guidelines and classifications of these chronic diseases. Although CRS most commonly has an allergic aetiology, there appears to be a gap in the literature and a lack of case studies that focus on fungal rhinosinusitis (FRS) and the different treatments for the various classifications of these fungal diseases 2. Classifications and treatments of FRS are constantly being improved. Current classification of FRS is divided into two groups: invasive and non-invasive 3. Further categorisation of FRS is done by subdividing both the non-invasive and invasive forms into three subgroups depending on the pathophysiology of the disease. Non-invasive FRS forms are: 1) saprophytic fungal infestation, 2) fungal ball, 3) allergic fungal rhinosinusitis (AFRS). Invasive FRS forms are the following: 1) acute invasive fungal rhinosinusitis, 2) chronic invasive fungal rhinosinusitis, 3) chronic granulomatous invasive fungal rhinosinusitis. The first two subgroups of non-invasive FRS can be undiagnosed and untreated for a long time; however, all other forms are in most cases treated with endoscopic sinus surgery (ESS). The use of antifungal agents is recognised in the treatment of the invasive forms of FRS, while treating non-invasive forms have shown little effect and stressed the need for ESS as a necessary treatment 4. There is a small number of case studies identifying fungal species in different forms of this disease. The positive outcome of the treatments vary across geographical regions as the isolation of fungal species is not always successful 5-7. The most common microorganisms are: Aspergillus, Mucor, Candida, Scedosporium and Penicillium 8.
The objective of this study was to analyse the aetiology, clinical presentations, histopathology and microbiology diagnostic aspects of FRS in 26 patients who underwent ESS.
Materials and methods
This descriptive study was performed from 2018 to 2022 in two hospitals: Otorhinolaryngology and Maxillofacial Surgery Clinic, University Clinical Center of Serbia and Otorhinolaryngology Clinic, University Medical Center “Zvezdara”, Belgrade, Serbia. The patients for the ESS procedure were chosen among those who experienced nasal obstruction, hypersecretion, hyposmia, anosmia, facial pain, and headache and were resistant to medical therapy. Patients with intraoperative endoscopic suspicion or CT signs of fungal sinus disease and pathohistological or microbiological fungal identification were selected to undertake ESS and take part in the case study. Patients who underwent ESS and had non-fungal related diseases were not included in the study. Preoperative CT was done a few weeks before surgery in order to assess sinus involvement, ostiomeatal complex, bone erosion or sinus cavity expansion. In most cases unenhanced CT was performed while contrast enhanced CT was chosen in cases with suspected bone erosion or endocranial involvement. Images were used during surgery for a safe endoscopic approach. In cases with suspected endocranial involvement, an MRI was performed. Although MRI and CT have similar sensitivity in detecting malignancy in sinuses, MRI demonstrates improved specificity compared to CT. MRI was not done in all cases with unilateral involvement due to higher costs and the reduced availability of such an examination. Enhanced CT was in most cases sufficient to provide correct information for a safe approach and correct diagnosis and treatment with ESS, while MRI was reserved for complicated cases. Nasal endoscopy was performed a day before surgery in order to visualise and diagnose signs of diseases including presence of polyps, oedematous mucosa, mucopurulent discharge and inspissated secretions. Surgery in most cases involved wide maxillary antrostomy and complete ethmoidectomy. In significantly fewer cases, depending on the degree of involvement of the sinuses, frontal and sphenoid sinus surgery was performed. ESS was performed using rigid endoscopes under general anaesthesia with the principles of removal of the entire lesion, polypoid mucosa, and secretions. An angled suction tube was used for repeated high-pressure washing of maxillary sinuses for clearance of all residual fungal materials. Specimens containing removed tissue, polyps and secretions were collected during surgery and sent for both microbiological and pathohistological analysis. Each specimen was observed under light microscopy using 10% potassium hydroxide and lactophenol cotton blue staining procedure followed by inoculation into Sabouraud’s Dextrose Agar (SDA) and Potato Dextrose Agar (PDA). For fungal culture, specimens were incubated at 26°C and 37°C for 4 weeks, while identification was done according to morphological characteristics of isolated fungi. Material sent for histopathological analysis was routinely processed and standard haematoxylin and eosin slides were made (Fig. 1, Cover figure); if needed, Grocott silver stain was also performed (Fig. 2). Histopathological examination was carried out to assess allergic mucin, fungal elements, presence of polyposis and the invasiveness of the infection. Nasal packing was done in cases with extensive bleeding and removed within 48-72 hours. Postoperative suction and crust removal was done on the second and seventh postoperative day in order to maintain good aeration of sinuses. Hypertonic saline douching was advised 3 times daily during the postoperative period. All patients were advised to use intranasal corticosteroids for 3 months after surgery. Regular follow-ups were done at 1, 3, and 6 months after surgery. The data were collected and analysed using the IBM SPSS Statistics, version 21.0 (IBM Corp., Armonk, NY, USA).
Results
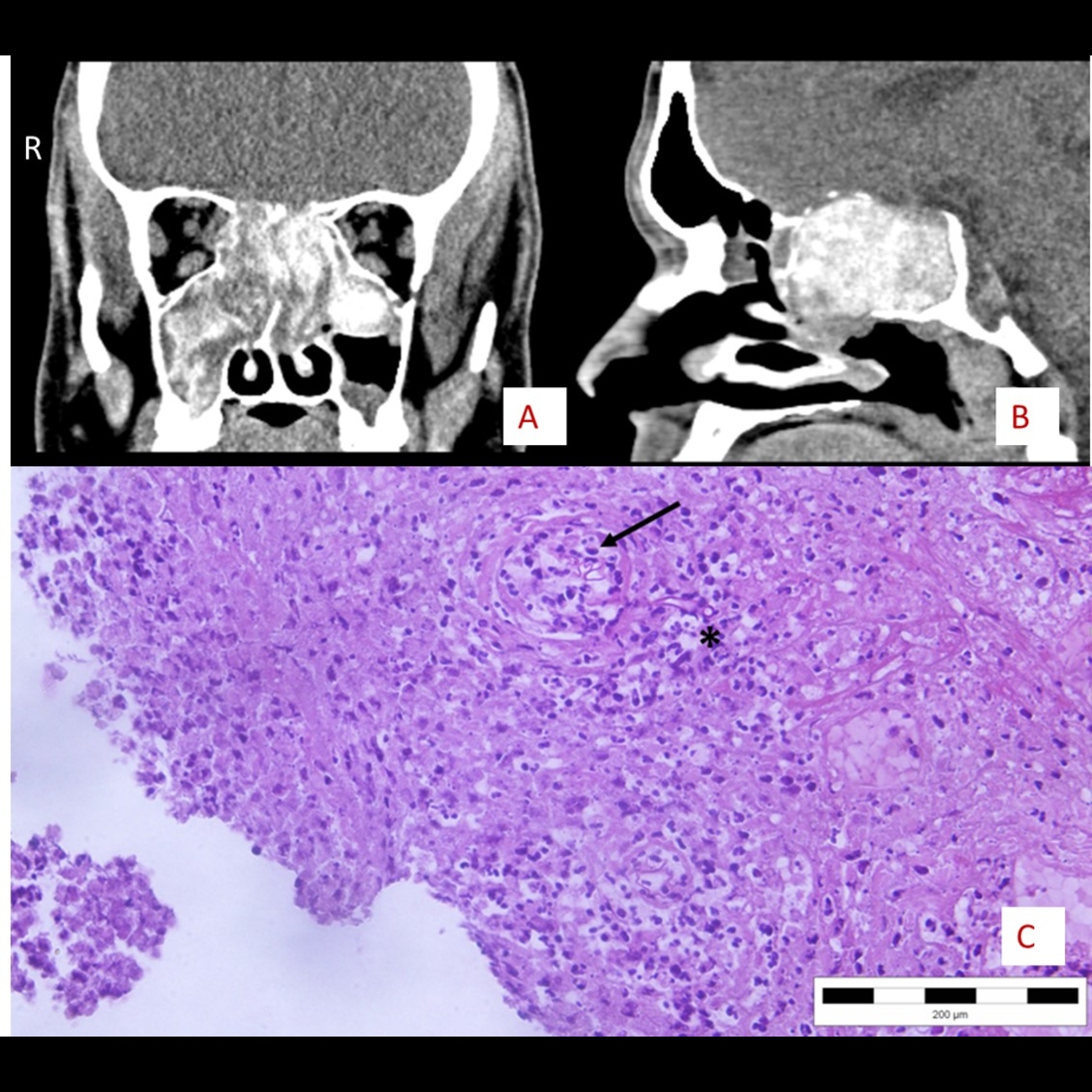
Twenty-six cases were included in this study during a 4-year period. There were 14 (53.8%) females and 12 (46.2%) males. The youngest patient was 24 years old, and the oldest 74 years. The mean age of patients was 40 years. Nearly half (46.1%) were in their fourth decade of life. A detailed age and gender distribution is shown in Table I. There were 4 patients (15.4%) with a previous history of ESS, of which 2 (7.7%) had undergone ESS twice. Among the associated risk factors, 8 patients (31%) had allergies, 4 (15.3%) asthma, and 2 (7.7%) diabetes mellitus type II. Except patients with diabetes who belonged to the saprophytic fungal infestation group, all the other patients were in the AFRS group. Symptoms of all patients lasted from 3 to 24 months prior to surgery, and all were treated with intranasal corticosteroids for a minimum of 2 months prior to surgery. The common symptoms were nasal obstructions in 24 patients (92.3%), followed by hypersecretion in 22 (84.6%), hyposmia in 20 (77%), and anosmia in 10 (38.5%). The frequency of symptoms is shown in Table II. Nasal endoscopy done before surgery revealed the presence of polyps in all patients. Oedematous mucosa was found in all patients and mucopurulent discharge in 19 (73%). CT done preoperatively revealed bilateral sinus involvement in 6 patients (23%). In cases with unilateral ethmoid and maxillary sinus involvement, there was one case of expansion of the involved sinus, 3 cases of remodelling and thinning of the bone sinus wall and 2 cases of its erosion. Radiologic characteristics of the AFRS subgroup and involvement of different sinuses is shown in Table III. In one case of saprophytic fungal infestation, the pathohistological finding did not reveal the presence of fungi, even though microbiological direct preparation was positive, and the culture showed that Schizophyllum commune was present. In 4 cases, pathohistology demonstrated fungal elements, direct preparations were positive and microbiological culture was negative. Negative microbiological findings with positive pathohistological identification were present in 5 cases, of which 4 were AFRS. The most common form of disease was AFRS in 18 cases (69.2%), followed by saprophytic fungal infestation in 4 (15.4%) and fungal ball in 3 (11.5%) (Tab. IV). Details of fungal species identified in each FRS type are shown in Table V. In one case of chronic granulomatous invasive fungal rhinosinusitis, Madurella sp. was identified according to fungal morphology in direct preparation from Sabourad culture and proven by matrix assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF MS) (Fig. 3). Preoperative CT in most of the cases showed the specific signs of double density opacification inside sinuses. Bone erosion of sinus walls was better observed in CT bone images, while the double density is visible in the soft tissue image series (Fig. 4A-B, Cover figure). In case of chronic granulomatous invasive fungal rhinosinusitis MRI with contrast was performed in order to assess the endocranial involvement and type of tissue causing bone erosion in different image sequences (Fig. 4C-D). During follow-up examinations, 5 patients (19.2%) had additional controls for crust removal between the seventh day and one month regular follow-up period. Seven patients stopped using nasal corticosteroids after one month arbitrarily. There were signs of disease relapse in 2 patients, in one after 6 months and in the other one after 9 months postoperatively. Disease relapse occurred in the patients with AFRS who stopped using nasal steroids at one month after surgery. Examinations at 6 and 9 months after surgery revealed the bilateral presence of nasal polyps in the middle meatus. The patients had symptoms of hypersecretion only, and the symptoms were well controlled after continuation of the nasal steroids.
Discussion
Different types of fungal rhinosinusitis found in our study show how important collaboration of otorhinolaryngologists with pathologists and microbiologists is in order to correctly diagnose and treat patients. Each type of fungal rhinosinusitis will be discussed separately due to the differences of each entity.
Saprophytic fungal infestation
Saprophytic fungal disease is characterised by fungal colonization of one or more paranasal sinuses in an immunocompetent host 9. Typically confined to a small area within a single sinus cavity provoking minimal underlying inflammatory changes, there is also speculation that it can be the starting point for development of fungal ball 10. Our study included 4 cases who were operated on due to symptoms of nasal obstruction in 75% and hypersecretion in all cases. These patients are the tip of the iceberg in such a of disease because mucous crusts within the nasal cavity are referred as the main symptom by most authors 11. Saprophytic fungal infestation is a form of disease that in some cases can be treated non-surgically with nasal douching, and does not require formal surgical intervention 3. Patients in this study had fungal presence and nasal polyposis in 3 of 4 cases and one patient had previous dental intervention on the left maxillary sinus. Presence of diabetes mellitus type II as a concomitant disease in one of 4 patients was not observed as a predisposing factor for this entity due to the lack of literature data, and the small number of patients in our study to form such a conclusion. Preoperative CT in 25% of the cases showed the specific signs of double density opacification inside sinuses, and mucosa thickening was seen in all the patients, and we thus conclude that there was no specific CT sign for this FRS entity. This form of FRS has been least described in the literature, and we did not see any findings that could be correlated. The principles of surgery in all cases is to clear all involved sinuses of fungal debris and oedematous mucosa similar to other forms of FRS. In our study we used local steroids postoperatively in order to reduce mucosa swelling and allow adequate sinuses drainage in all cases regardless of the type of FRS. The fungal identification rate of 50% is similar to other studies 12. Because of the limitation of this study regarding inclusion criteria and the small number of patients with saprophytic fungal infestation, it is hard to conclude that this group represents the starting point for fungal ball development as hypothesised by some authors 13.
Fungal ball
The fungal ball form of the disease has been seen more often in the last decade due to refinements in radiologic evaluation and more common use of ESS. The percentage of fungal ball cases to total cases of ESS is around 10%, but due to nonspecific clinical presentations and asymptomatic cases it ranges from 6 to 13.2% among operated patients; we can only estimate that the proportion is actually much higher 14. Considering that our study included only operated patients with FRS, our frequency of 11.5% is similar to that reported by other authors. Fungal isolation in fungal ball cases reported by the same author in 669 samples is only 22.6% due to problems with culture and shows why in our 3 cases Botrytis spp was isolated in only one patient 14. Identification by histological examinations performed before culturing as seen in the study performed by Fadda et al. can increase the percentage to 77.5% 15. Dominant symptoms and predominant unilateral and maxillary sinus involvement seen in the literature is similar to our findings in all 3 cases 16. Even if concomitant diseases were not recorded in this study, probably due to small number of patients, it is worth mentioning that fungal ball has been occasionally reported in combination with other pathologies, but these associations are considered incidental by some authors 16. According to Fadda et al., a previous history of endodontic treatment was an important predisposing factor for the development of maxillary fungal ball in 46.1% of cases 15. In our 3 cases, 2 had nasal polyposis with ethmoidal and maxillary involvement, but the fungal ball was always located in the maxillary sinus. Radiological findings of all patients were typical as seen in literature showing opacification in involved sinus appearing as a metal-dense spot. ESS lead to resolution of symptoms in all patients during follow-up. Data from the literature suggest ESS as a treatment of choice for paranasal sinus fungal ball (PSFB), even if further prospective, and hopefully randomised, study of the ancillary procedures and treatments is needed which might lead to the reduction of unnecessary further antibiotic treatments and revision surgeries and a better management of PSFB patients 17. Any contributing factors (i.e., oroantral fistula or retained dental amalgam) should also be treated 3. Antifungal treatment is not effective, but oral or local steroid treatments should be used in order to maintain aeration of sinuses. In some studies, there is a certain group of immunosuppressed and symptomatic patients in whom antifungal therapy may provide some benefits and disease control 15. In these patients, high levels of β-D-glucan antigen in blood can be seen by serological testing and CT/MRI indicates the presence of PSFB 15.
Allergic fungal rhinosinusitis
The diagnostic criteria of AFRS focus on the combination of characteristic clinical, radiographic, histopathologic findings and immunologic characteristics of the disease. It has been 25 years since Bent and Kuhn outlined the diagnostic criteria for AFRS: 1) type I hypersensitivity, 2) nasal polyposis, 3) characteristic CT appearance, 4) eosinophilic mucus, and 5) presence of non-invasive fungus in sinus contents 11,18. Multiple sets of criteria have been proposed over time for diagnosis of AFRS and some authors recognise national and regional variations. De Shazo and Swaim have also proposed more or less similar diagnostic criteria, with the exception of atopy, and Cody proposed determination of fungal specific IgE and IgG levels in cases with absence of positive culture or hyphae identification 19-21. Further immunological research of AFRS summarised by Matthew et al. showed that the presence of fungus, IgE and systemic hypersensitivity to fungal antigens, and presence of positive fungal cultures was seen in both non-AFRS and healthy patients and additionally, reactivity to fungal antigen and the presence of fungal-specific IgE and IgG could be demonstrated in both non-allergic and AFRS 22. Pant et al. demonstrated that fungal-specific IgE levels were not significantly different in patients with AFRS and fungal-specific peripheral blood lymphocytes could be observed in both AFRS and CRS without fungal allergy 23. Terms like eosinophilic fungal rhinosinusitis (EFRS) and eosinophilic mucin rhinosinusitis (EMRS/EFRS-like) for diseases without type I hypersensitivity have only led to greater confusion in categorisation 11. One of the problems in Serbia in daily practice is conducting type I hypersensitivity tests in patients to comply with the Bent and Kuhn criteria of diagnosing AFRS. Increased fungal specific IgE is not possible to diagnose in all hospitals due to lack of standard commercially available tests on the domestic market and insurance coverage in public hospitals. Diversity of fungal isolation would be a challenge for routine testing in all hospitals since fungal PRICK testing is done in selected laboratories in our country. Despite improving our understanding of the biological basis of AFRS, the diagnosis of this disease remains largely clinical and relies on clinicopathological, radiological and microbiological correlations. The most common symptoms seen in our study were nasal obstruction, hypersecretion and hyposmia and had similar incidences in all forms of FRS, although nasal discharge in AFRS patients was much thicker, with patients often reporting its glue-like consistency before surgery. Comorbidities in patients from our study do not differ from those reported in the literature 18. In our study, unenhanced CT was done routinely and showed that sinuses were opacified by centrally hyperdense material with a peripheral rim of hypodense mucosa in 16 cases. Comparing the literature, approximately 40% of patients may have each of the following signs: expansion of an involved sinus, remodelling, thinning or erosion of the bone sinus wall 24. In our study there were 8 patients (30.8%) with those signs, which is similar to literature data (Tab. III). In our study, ESS in the AFRS group is more challenging due to the nature of disease. Care must be taken to avoid any inadvertent injury to critical structures such as the optic nerve, carotid artery, dura, etc., which could have become dehiscent secondary to bone resorption. AFRS patients are reported to be 12 times more likely to have bony dehiscence than non-AFRS patients needing surgery 25. In our study, there were no complications recorded, but in revision surgery special attention should be taken. The microbiology identification rate in the AFRS group was 72.2%, which is higher than in other non-invasive FRS groups. Fungal rich mucin sent for analysis may explain these results, although all histopathological direct preparations revealed fungal presence. Fungal isolation is challenging in everyday practice, and possibly the reason for low rate of fungal isolation is the material and the way it is sent for analysis. During our study, we observed a higher identification rate over time as we aimed to reach the mucus, and shortening the time lapse of sending material for microbiology. Due to high use of suction in ESS, fungus rich mucus is removed with blood and debris and becomes unavailable for analysis. A possible solution for this problem would be routine use of more sensitive methods like single cell suspension 26. Out of 13 patients in the AFRS group who had fungal species identified, 2 had multiple fungi isolated. One patient had isolation of Alternaria spp., Penicillium spp. and Stemphylium spp, and the second had Cladosporium spp. and Penicillium spp. cultures. Alternaria spp. and Cladosporium spp. are often seen in AFRS, and Penicillium could be described as a contaminate. Stemphylium spp. is interesting as it has not often been described and is closely related to Alternaria and recent taxonomy places it as a sister clade to the latter and polyclonal antibodies to Alternaria alternata cross-react broadly to Stemphylium and Cladosporium 27. In the literature, the most common fungi for this type of disease are Aspergillus species, dematiaceous moulds (Bipolaris, Curvularia more common than Alternaria) and hyaline moulds (Paecilomyces, Fusarium, Scedosporium) 18. In our study, Cladosporium spp. was identified in 5 patients (38.5% of isolated) and dematiaceous moulds in same number, while the rest of the cultures were hyaline moulds. There were no cases of Aspergillus spp., which could be explained by regional differences and the small number of patients included, but nonetheless warrants attention for further research.
When we look at the microbiology results of other studies, there are large regional differences where Aspergillus spp. isolation ranges from 13 to 94.2% 5,6,28,29. Treatment options of AFRS are debated since the discovery of this form of disease. Due to association with orbital involvement and the high rate of early recurrence in non-surgically treated patients, surgery in the form of full clearance of the fungal material and mucin as well as restoration of sinus drainage pathways is key 3. Creating a wide opening for all sinuses in order to improve ventilation, as well as allowing a pathway for ongoing postoperative topical therapy to the sinus cavities also allowed for long-term in-office endoscopic examination to detect early recurrence of disease and allow timely management. Incomplete debridement has been linked to early recurrence of the disease and the need for revision surgery 18. In our study, ESS usually consisted of a complete ethmoidectomy with a wide maxillary antrostomy and frontosphenoidectomy was done according to findings of preoperative CT or during surgery.
Medical therapy is integral for successful treatment of AFRS and in our practice we used local and oral steroids, antifungal medications, and leukotriene modulators. A variety of studies is also available on the use of omalizumab and immunotherapy. Topical rinses aim to improve inflammation, infection and mucociliary dysfunction which accompanies the disease process 25. In our study hypertonic saline douching was advised accordingly.
The mainstay of postoperative medical treatment proposed by most authors is routine use of oral and topical steroids 3,30. Oral steroid treatment has shown benefit in postoperative mucosal disease, but because of the potential side effects of long-term therapy the dose and duration of oral steroids should be tailored to the patient’s disease and risk of recurrence. Some authors proposed that oral steroids, with all their concomitant side effects, should be reserved only for patients with severe SNOT-22 scores along with pulmonary worsening during acute exacerbations in the post-surgical period and we agree on that position 25. We do not advise use of oral steroids because of potential side effects and adequate control of disease was seen in all but 2 cases who had recurrence after acute rhinosinusitis. Those patients were candidates for oral treatment.
In comparison to the CRS with nasal polyps scenario, patients with AFRS should be candidates for more frequent controls and therapies with both local and short-term oral steroids. By literature review, although the evidence for the use of standard and nonstandard topical nasal steroids is lacking for AFRS, expert opinion would support that this form of medical treatment is a safe and viable option 30. In our study we used local steroids postoperatively and by our estimation they showed benefits without risks.
There are only a few reports that have described the benefit of oral antifungals in patients with refractory AFRS, but a Cochrane review of topical and systemic antifungal therapies in patients with all phenotypes of CRS did not demonstrate any clinical benefit 18. The use of oral antifungals does not seem to drastically improve symptom or radiological scores, but could be considered in some recalcitrant cases as adjunctive therapy together with topical steroids 25. Topical antifungals were eventually abandoned as a treatment for AFRS due to ineffectiveness and side effects. In our study, antifungal treatment was not used for treatment of AFRS. Advanced immunotherapy and biologics are still unavailable in our country to treat AFRS. Current literature data show potential benefits. Immunotherapy has emerged as a promising method based on greater understanding of the pathophysiology of AFRS. In one study fungal antigen immunotherapy combined with surgery showed a significant reduction in the production of allergic mucin, fungal debris and crusts, and reduced use of intranasal steroids that completely negated the need for systemic steroids 25. With advances in understanding the immunological nature of AFRS, biologics have emerged as a potential therapy. Biologic agents such as mepolizumab or omalizumab has been successfully used in patients with asthma. In a selected group of patients with resistant AFRS concomitant with asthma, dupilumab therapy has shown benefit. Long-term follow-up has demonstrated that dupilumab improved symptoms and objective measurements such as SNOT-22, imaging, and pulmonary function 31. It will be interesting to see the results of randomised control trials in the near future.
Chronic granulomatous invasive fungal rhinosinusitis
Chronic granulomatous invasive fungal rhinosinusitis is very rare in our country due to its continental climate, but imported cases can be seen from time to time.
In case of chronic granulomatous invasive fungal rhinosinusitis, it is interesting that one patient had a history of fungal sinus disease and ESS. The first operation was 2 years after the first symptoms that occurred a couple of months after a trip to Egypt. The patient had no comorbidities and was 42 years old. The first symptoms were hypersecretion and periodic nose congestion, but the patient ignored the symptoms for 2 years. The first medical examination was due to visual disturbance and constant nose blockage. The first operation revealed a noninvasive fungal form of disease without identification of fungus and 2 months after the first operation a reoperation was performed due to disease relapse, and the patient was included in the present study group. Chronic granulomatous invasive fungal rhinosinusitis was diagnosed after the patient’s second operation. Preoperative CT and MRI showed the dominantly ethmoidal and sphenoidal involvement that is often seen in studies conducted by authors with more experience in this form of FRS 32. ESS was performed with a neurosurgery team stand-by due to the extensive nature of the process which partially destroyed the walls of sphenoidal and maxillary sinuses, but no complications were recorded. After the second operation, direct preparation and culture showed Madurella spp., which was confirmed by MALDI-TOF MS (Fig. 5). The method of identification is not in routine use, but offers great possibilities for identification. In cases like this, where there is only one case in literature with this fungi species invading sinuses, it is very important to have a correct diagnosis 33. This case also shows possibility of the disease to change from a noninvasive to invasive form. Treatment of this patient was continued with consultation of infectious disease physicians who prescribed amphotericin B, which lead to the patient’s full recovery.
Conclusions
Understanding the diversity of the fungal forms of rhinosinusitis has been improved in last decades. Regardless of the range of methods, the process of diagnosing FRS is still extremely complex and challenging. Difficulties in diagnosis are caused by discordance between microbiological and pathohistological confirmation of the infection itself, clinical findings, and CT imaging, which varies in FRS entities. Based on our data, the most common form of FRS treated by ESS is AFRS. The fungus identification rate varies between entities, and was 72.2% in AFRS and 33.3% in fungal ball. The most common species seen in AFRS isolates in this study were Cladosporium spp. (38.5% of isolated) and dematiaceous moulds in same percentage, while the rest of the cultures were hyaline moulds. These findings represent our region in this number of patients, which differ from studies in other regions and should be the basis for further research. CT can be very helpful in diagnosing FRS and even in differentiating between different entities. Treatment of FRS should be adjusted for each entity. Postoperative medical treatment in AFRS should be personalised given the potential advancements described in literature. More frequent control visits and adequate therapy can help to prevent relapses. Our study emphasises the need to combine all types of clinical, radiological, pathohistological and microbiological methods to optimise diagnostic and treatment strategies and should be the basis for further research.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
VM, SC: data collection, conceptualisation, design, writing, review and editing; AT: validation, review, supervision; IĆČ, JM, SB, NT: data processing, review of the manuscript; ZR: editing, final review of the manuscript.
Ethical consideration
This study was approved by the Institutional Ethics Committee (Research and Ethics Committee of the Zvezdara University Medical Center, Belgrade, Serbia) (Approval number: 5/8/23). The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki. Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: November 8, 2023
Accepted: July 21, 2024
Published online: January 15, 2025
Figures and tables
Figure 1. Pathohistological changes in allergic rhinosinusitis. H&E staining. Magnification, x200.
Figure 2. Fungal organisms are highlighted by Grocott methenamine silver stain. Magnification, x400.
Figure 3. Appearance of fungal hyphae in granuloma. Grocott methenamine silver stain. Magnification, x400.
Figure 4. Radiological preoperative images. A) CT image in bone window, B) CT image in soft tissue window showing characteristic double density, C) MR image in S3DI/angio-sense mode, D) MR image in T2W mode.
Figure 5. Pathohistological appearance of fungal hyphae surrounded by eosinophil infiltrate and giant cells. H&E staining. Magnification, x400.
| Age (years) | N/Percentage | Gender | |
|---|---|---|---|
| Male (46.2%) | Female (53.8%) | ||
| 21-30 | 4/15.4% | 2 | 2 |
| 31-40 | 12/46.1% | 4 | 8 |
| 41-50 | 2/7.7% | 2 | / |
| 51-60 | 5/19.2% | 2 | 3 |
| 61-70 | 0 | / | / |
| 71-80 | 3/11.5% | 1 | 2 |
| Symptoms | Number of patients | Percentage (%) |
|---|---|---|
| Nasal obstruction | 24 | 92.3 |
| Hypersecretion | 22 | 84.6 |
| Hyposmia | 20 | 77 |
| Anosmia | 10 | 38.5 |
| Facial pain | 9 | 34.6 |
| Headache | 4 | 15.4 |
| Involved sinus | Complete opacity | Partial opacity | Hyperdense material | Expansion of an involved sinus | Remodelling and thinning of the bony sinus wall | Erosion of the sinus wall |
|---|---|---|---|---|---|---|
| MS (unilateral) | 2 | |||||
| MS+ES (unilateral) | 6 | 2 | 1 | 1 | 3 | 2 |
| MS+ES (bilateral) | 1 | 1 | 2 | |||
| MS+ES+SS (unilateral) | 1 | |||||
| MS+ES+FS (bilateral) | 2 | 1 | ||||
| MS+ES+FS+SS (bilateral) | 1 | |||||
| MS: maxillary sinus; ES: ethmoid sinus; SS: sphenoid sinus; FS: frontal sinus. | ||||||
| Number of patients | Percentage of patients | Fungus identification rate | |
|---|---|---|---|
| Saprophytic fungal infestation | 4 | 15.4 | 50% |
| Fungal ball | 3 | 11.5 | 33.3% |
| Allergic fungal rhinosinusitis | 18 | 69.2 | 72.2% |
| Chronic granulomatous invasive fungal rhinosinusitis | 1 | 3.9 | 100% |
| Saprophytic fungal infestation | Fungal ball | Allergic fungal rhinosinusitis | Chronic granulomatous invasive fungal rhinosinusitis | |
|---|---|---|---|---|
| Schizophyllum commune | 1 | |||
| Penicillium spp. | 1 | |||
| Botrytis spp. | 1 | |||
| Culvularia spp. | 2 | |||
| Scedosporium apiospermum | 2 | |||
| Penicillium spp. | 2 | |||
| Cladosporium spp. | 2 | |||
| Chrysosporium spp. | 1 | |||
| Alternaria spp. | 2 | |||
| Bipolaris spp. | 1 | |||
| Stemphylium spp. | 1 | |||
| Madurella spp. | 1 |
References
- Beule A. Epidemiology of chronic rhinosinusitis, selected risk factors, comorbidities, and economic burden. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2015;14. doi:https://doi.org/10.3205/cto000126
- Rodrigues M, Nosanchuk J. Fungal diseases as neglected pathogens: a wake-up call to public health officials. PLoS Negl Trop Dis. 2020;14. doi:https://doi.org/10.1371/journal.pntd.0007964
- Deutsch P, Whittaker J, Prasad S. Invasive and non-invasive fungal rhinosinusitis – A review and update of the evidence. Medicina (Kaunas). 2019;55. doi:https://doi.org/10.3390/medicina55070319
- Ebbens F, Georgalas C, Luiten S. The effect of topical amphotericin B on inflammatory markers in patients with chronic rhinosinusitis: a multicenter randomized controlled study. Laryngoscope. 2009;119:401-408. doi:https://doi.org/10.1002/lary.20064
- Manning S, Holman M. Further evidence for allergic pathophysiology in allergic fungal sinusitis. Laryngoscope. 1998;108:1485-1496. doi:https://doi.org/10.1097/00005537-199810000-00012
- McClay J, Marple B, Kapadia L. Clinical presentation of allergic fungal sinusitis in children. Laryngoscope. 2002;112:565-569. doi:https://doi.org/10.1097/00005537-200203000-00028
- Suresh S, Arumugam D, Zacharias G. Prevalence and clinical profile of fungal rhinosinusitis. Allergy Rhinol (Providence). 2016;7:115-120. doi:https://doi.org/10.2500/ar.2016.7.0156
- Waghray J. Clinical study of fungal sinusitis. Int J Otorhinolaryngol Head Neck Surg. 2018;4:1307-1312. doi:https://doi.org/10.18203/issn.2454-5929.ijohns20183707
- Tyler M, Lam K, Marino M. Revisiting the controversy: the role of fungi in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2021;11:1577-1587. doi:https://doi.org/10.1002/alr.22826
- Ferguson B. Definitions of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:227-235. doi:https://doi.org/10.1016/S0030-6665(00)80002-X
- Chakrabarti A, Denning D, Ferguson B. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope. 2009;119:1809-1818. doi:https://doi.org/10.1002/lary.20520
- Zuluaga A, Ospina-Medina J, Castaño-Gallego I. Frequency of fungal agents identified in sinus samples from patients with clinically suspected rhinosinusitis. Diagn Microbiol Infect Dis. 2015;81:208-212. doi:https://doi.org/10.1016/j.diagmicrobio.2014.11.017
- Montone K. Pathology of fungal rhinosinusitis: a review. Head Neck Pathol. 2016;10:40-46. doi:https://doi.org/10.1007/s12105-016-0690-0
- Liu X, Liu C, Wei H. A retrospective analysis of 1,717 paranasal sinus fungus ball cases from 2008 to 2017. Laryngoscope. 2020;130:75-79. doi:https://doi.org/10.1002/lary.27869
- Fadda G, Succo G, Moretto P. Endoscopic endonasal surgery for sinus fungus balls: clinical, radiological, histopathological, and microbiological analysis of 40 cases and review of the literature. Iran J Otorhinolaryngol. 2019;31:35-44.
- Costa F, Emanuelli E, Franz L. Fungus ball of the maxillary sinus: retrospective study of 48 patients and review of the literature. Am J Otolaryngol. 2019;40:700-704. doi:https://doi.org/10.1016/j.amjoto.2019.06.006
- Fadda G, Allevi F, Rosso C. Treatment of paranasal sinus fungus ball: a systematic review and meta-analysis. Ann Otol Rhinol Laryngol. 2021;130:1302-1310. doi:https://doi.org/10.1177/00034894211002431
- Dykewicz M, Rodrigues J, Slavin R. Allergic fungal rhinosinusitis. J Allergy Clin Immunol. 2018;142:341-351. doi:https://doi.org/10.1016/j.jaci.2018.06.023
- Al-Qahtani K, Altamimi F, Al-Harbi M. The evaluation of the sensitivity and specificity of a new endoscopic diagnostic sign of allergic fungal rhinosinusitis: intrapolypoidal white particles. J Maxillofac Oral Surg. 2021;20:612-618. doi:https://doi.org/10.1007/s12663-020-01357-4
- Cody D, Neel H, Ferreiro J. Allergic fungal sinusitis: the Mayo Clinic experience. Laryngoscope. 1994;104:1074-1079. doi:https://doi.org/10.1288/00005537-199409000-00005
- Chakrabarti A, Kaur H. Allergic aspergillus rhinosinusitis. J Fungi (Basel). 2016;2. doi:https://doi.org/10.3390/jof2040032
- Tyler M, Luong A. Current understanding of allergic fungal rhinosinusitis. World J Otorhinolaryngol Head Neck Surg. 2018;4:179-185. doi:https://doi.org/10.1016/j.wjorl.2018.08.003
- Pant H, Beroukas D, Kette F. Nasal polyp cell populations and fungal-specific peripheral blood lymphocyte proliferation in allergic fungal sinusitis. Am J Rhinol Allergy. 2009;23:453-460. doi:https://doi.org/10.2500/ajra.2009.23.3356
- Mukherji S, Figueroa R, Ginsberg L. Allergic fungal sinusitis: CT findings. Radiology. 1998;207:417-422. doi:https://doi.org/10.1148/radiology.207.2.9577490
- Medikeri G, Javer A. Optimal management of allergic fungal rhinosinusitis. J Asthma Allergy. 2020;13:323-332. doi:https://doi.org/10.2147/JAA.S217658
- Barac A, Pekmezovic M, Spiric V. Chronic rhinosinusitis: association of recalcitrant nasal polyposis and fungal finding in polyp’s single-cell suspension. Eur Arch Otorhinolaryngol. 2015;272:3727-3734. doi:https://doi.org/10.1007/s00405-015-3511-2
- Weber R. Allergen of the month – Stemphylium. Ann Allergy Asthma Immunol. 2015;114. doi:https://doi.org/10.1016/j.anai.2014.11.008
- Ponikau J, Sherris D, Kern E. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877-884. doi:https://doi.org/10.4065/74.9.877
- Kaur R, Lavanya S, Khurana N. Allergic fungal rhinosinusitis: a study in a Tertiary Care Hospital in India. J Allergy (Cairo). 2016;2016. doi:https://doi.org/10.1155/2016/7698173
- Gan E, Thamboo A, Rudmik L. Medical management of allergic fungal rhinosinusitis following endoscopic sinus surgery: an evidence-based review and recommendations. Int Forum Allergy Rhinol. 2014;4:702-715. doi:https://doi.org/10.1002/alr.21352
- Bulkhi A, Mirza A, Aburiziza A. Dupilumab: an emerging therapy in allergic fungal rhinosinusitis. World Allergy Organ J. 2022;15. doi:https://doi.org/10.1016/j.waojou.2022.100638
- Fadda G, Martino F, Andreani G. Definition and management of invasive fungal rhinosinusitis: a single-centre retrospective study. Acta Otorhinolaryngol Ital. 2021;41:43-50. doi:https://doi.org/10.14639/0392-100X-N0848
- Fahal A, Mahgoub E, El Hassan A. Head and neck mycetoma: the mycetoma research centre experience. PLoS Negl Trop Dis. 2015;9. doi:https://doi.org/10.1371/journal.pntd.0003587
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2024 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1640 times
- PDF downloaded - 204 times



