Audiology
Vol. 44: Issue 5 - October 2024
Up-regulation of HIF-1α in refractory sudden sensorineural hearing loss
Abstract
Objectives. To investigate the expression of hypoxia-inducible factor-1α (HIF-1α) in patients with refractory sudden sensorineural hearing loss (SSNHL).
Material and methods. Thirty patients with refractory SSNHL were treated with intratympanic methylprednisolone perfusion (IMP) for 10 days. Expression of HIF-1α and histone deacetylase 2 (HDAC2) was evaluated in peripheral blood mononuclear cells (PBMCs) and in vitro.
Results. Significant hearing improvement (≥ 15 dB) was observed in 16 patients [IMP glucocorticoid sensitive (GCS) group], while 14 patients had no therapeautic hearing recovery [IMP GC resistance (GCR) group]. The expression of HDAC2 decreased and HIF-1a increased in all refractory SSNHL patients before IMP. The expression of HDAC2 and HIF-1α after IMP was significantly changed in the GCS group, but not in the GCR group. The same expression profile was also observed in House Ear Institute-organ of Corti-1 (HEI-OC1) cells exposed to oxidative stress (OS). The results of gene manipulation experiments indicate that HIF-1α up-regulation significantly reduced HDAC2 expression in HEI-OC1 cells, especially under conditions of OS.
Conclusions. This study suggests that HIF-1α activation inhibits HDAC2 expression, causing glucocorticoid resistance in refractory SSNHL. HIF-1α might serve as a potential biomarker to predict prognosis of refractory SSNHL.
Introduction
Sudden sensorineural hearing loss (SSNHL) is defined as an idiopathic hearing loss of at least 30 dB over at least three consecutive frequencies occurring within 3 days 1. SSNHL can cause significant morbidity 2. Moreover, the incidence of SSNHL is increasing annually, and is estimated to affect 400 per 100,000 people worldwide 3. Although the aetiology of SSNHL has still not been identified, oxidative stress (OS) is recognised as a main pathological factor in SSNHL 4-7. Due to the effects of anti-inflammation and antioxidation, glucocorticoid (GC) is considered to be the most beneficial treatment for SSNHL 1,5,7-9. However, a growing amount of evidence indicates that 20% of SSNHL patients exhibit resistance, or no positive response, to systemic steroid treatment 10. The mechanisms of GC resistance, especially at the molecular level, are largely unknown. In our previous studies, reduced expression of histone deacetylase 2 (HDAC2) was reported to contribute to steroid resistance in SSNHL 11-13. In the body, the function of HDAC2 is to inhibit the transcription of inflammatory genes 14,15. A number of studies have demonstrated that OS plays an important role in GC resistance by inhibiting the expression and activity of HDAC2 14,15.
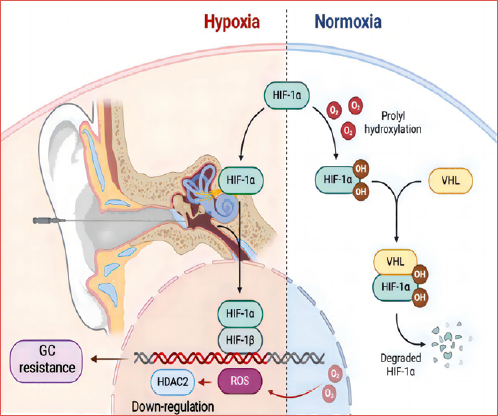
The transcription factor hypoxia-inducible factor-1 (HIF-1, a molecular determinant of responses to hypoxia) consists of two subunits: an oxygen-sensitive HIF-1α and a constitutively expressed HIF-1β. HIF-1α is a key nuclear factor in the cellular adaptive response to hypoxia 16. The stability and transcriptional activities of HIF-1α are precisely regulated by intracellular oxygen concentrations. Some studies have shown that the stabilisation of HIF-1α, mainly under inflammatory conditions, has important implications in chronic inflammatory diseases 17, 18. It has been demonstrated that overproduction of reactive oxygen species (ROS) results in corticosteroid-insensitive inflammation via reduced transcription of HDAC2 in models of chronic obstructive pulmonary disease in vitro 19. In our previous studies, we found that the prognosis of patients with refractory SSNHL is closely related to the level of HDAC2 in peripheral blood mononuclear cells (PBMCs) 11-13. Therefore, we speculated that OS in patients with SSNHL causes up-regulation of HIF-1α and down-regulation of HDAC2 and eventually GC resistance (Cover figure).
The value of peripheral blood biomarkers to predict GC resistance and prognosis of SSNHL is still unclear 1. Although several existing OS indexes suggested that OS is involved in the pathological process of SSNHL, these indexes might not be useful to predict the prognosis of SSNHL 6,8,11. In the present study, we prospectively investigated the efficacy of intratympanic methylprednisolone perfusion (IMP) treatment, and examined the expression of HIF-1α and HDAC2 in PBMCs from patients with refractory SSNHL before and after IMP treatment, as well as in House Ear Institute-organ of Corti-1 (HEI-OC1) cells to explore whether HIF-1α can change the expression of HDAC2 and understand its role in GC resistance in refractory SSNHL. We demonstrated that up-regulation of transcription factor HIF-1α is necessary and sufficient to reduce HDAC2 expression, resulting in GC resistance. Expression of HIF-1α in PBMCs could be used to predict the prognosis of patients with refractory SSNHL.
Materials and methods
Subjects
The study protocol was approved by the ethics committee of Nanjing Drum Tower Hospital (IRB2016-194-01), and informed consent forms were obtained from all participants. In the present study, 30 patients with refractory SSNHL (18-65 years old) who failed a conventional 7-10 day treatment were recruited from March 2017 to March 2019 in Nanjing Drum Tower Hospital, China. The conventional treatment included systemic dexamethasone (10 mg/day for 4 days and then 5 mg/day for 3 days, totaling for 7 days) or methylprednisolone (80 mg/day for 4 days, 40 mg/kg for 3 days and then 20 mg/kg for 3 days, totaling for 10 days) and injection of Ginkgo Biloba extract (EGB761, an antioxidant, 105 mg/day for 10 days). All patients had a PTA improvement less than 15 dB (0.25-8 kHz) after the conventional treatment 20. All patients had severe to profound hearing loss (PTA at 0.5-4 kHz > 60 dB) in the affected ears when recruited. Identifiable causes of hearing loss, such as acoustic neuroma and stroke, were ruled out by neurotologic examination and head magnetic resonance imaging. No patient had a history of ear disease or family history of hearing loss. Ten volunteers with normal audiograms were enrolled in the study to obtain normal reference levels of HIF-1α and HDAC2 in PBMCs.
Treatment and follow-up
All enrolled patients were treated with intratympanic infusion of methylprednisolone sodium succinate (IMP, methylprednisolone, 20 mg/day, Pfizer, Inc, USA) for 10 consecutive days. Ginkgo Biloba extract injection (an antioxidant, 105 mg/day, intravenously, Dr. Willmar Schwabe, GmbH & Co KG, Essen, Germany) and monosialotetrahexosylganglioside sodium (a neurotrophic drug, 40 mg/day, Qilu Pharmaceutical, China) were also given to each patient according to the Chinese Medical Association guidelines for SSNHL 11,20. After 10 days of IMP, all patients were prescribed Ginkgo Biloba tablets (40 mg, three times/day, Dr. Willmar Schwabe, GmbH & Co KG, Essen, Germany) and Mecobalam tablets (a form of vitamin B12, 0.5 mg, three times/day, Yangtze River Pharmaceutical Group) for 2 months and followed-up with PTA for 2 months or longer. According to hearing improvement at 3 months after onset, patients were assigned into the GC sensitive group (the GCS group, PTA gain ≥ 15 dB at 0.25-8 kHz) and the GC resistant group (the GCR group, PTA gain < 15 dB at 0.25-8 kHz) 20.
Collection of PMBCs
Peripheral blood was collected from all refractory SSNHL patients before and immediately after 10-day IMP treatment and normal controls. PBMCs were extracted according to the manufacturer’s instructions (Tianjing Haoyang Biological Manufacture Co., China). PBMCs were then dispensed into two Eppendorf tubes and stored at -80°C until RNA and protein extraction.
Cell culture
HEI-OC1 cells were obtained from Prof. Renjie Chai of Southeast University, China, and cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% foetal bovine serum (FBS; Sigma) at 37°C with 5% CO2.
Transfection of HEI-OC1 cells with HIF-1α small interfering RNA (siRNA)
HEI-OC1 cells were seeded into 6-well plates at a density of 105 cells/well. When cell growth reached 50-60% confluence, siRNA transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific, MA, USA). Cells without transfection were used as controls. Cells were then incubated in Opti-MEM I reduced serum medium (ThermoFisher Scientific, MA, USA) for 6 hours. The transfection efficiency of siRNA was monitored by observing the fluorescence of cells transfected with FAM-labeled scrambled scRNA (FAM-scRNA) 6 hours after transfection. After incubation at 33 °C with 5% CO2 for 24 hours, the cells were then treated with 0.8 mM H2O2 (detailed below) for another 24 hours. Three different HIF-1α siRNA and FAM-labeled scRNA were designed and purchased from Gene Pharma (Shanghai, China). The sequences of HIF-1α siRNA used in the present study were as follows: 5’- CCAUGUGACCAUGAGGAAATT-3’; 5’- GCCUAACAGUCCCAGUGAATT-3’; 5’- GCAGACCCAGUUACAGAAATT-3’ and 5’-UUCUUCGAACGUGUCACGUTT -3’.
Construction, transfection and detection of recombinant HIF-1α adenovirus
A cDNA fragment encoding HIF-1α (NM_010431) was subcloned (BamHI/AgeI digestion) into GV314-CMV-3Flag-GFP (GENECHEM, Fig. 1A) 21. The recombinant plasmids CMV-MCS-3FLAG-SV40-EGFP were transfected into 293T cells to obtain adenovirus prestocks 22. The recombinant adenovirus (Ad-HIF-1α, Fig. 1B) was purified using CsCl banding, followed by dialysis in 10 mM Tris-buffered saline with 5/50 mL glycerol. The virus titer was determined using HEK293A cells and the Adeno-X Rapid Titer Kit (Clontech). Adenovirus expressing GV314-CMV-Flag-GFP was used as a control (Ad-CTL) in the HIF-1α-overexpression experiments.
HDAC2 and HIF-1α expression was examined in Ad-HIF-1α-infected HEI-OC1 cells. First, HEI-OC1 cells were infected with Ad-HIF-1α or Ad-CTL (200 multiplicity of infection) and cultured for 48 hours. The culture medium that contained recombinant HIF-1α protein was collected for Western blot analysis to identify the protein by detecting the Flag tag. The infected cells were then incubated in fresh DMEM medium containing 5% FBS for 24 hours under normoxic conditions (21% O2), followed by another 24 hours incubation under OS condition (0.8 mM H2O2). HEI-OC1 cells were then collected and used for immunolabelling and Western blotting to detect GFP, HDAC2 and HIF-1α expression.
Detection of apoptosis in HEI-OC1 cells by flow cytometry
Apoptosis was detected using the Annexin V kit (BD, USA). Briefly, HEI-OC1 cells were treated with various concentrations (0.5, 0.8, 1, 2, 4 mM) of H2O2 for 24 hours. Cells without H2O2 treatment were used as the control group. After H2O2 treatment, cells were trypsinised, collected by centrifugation at 3000 × g for 5 min, washed twice with PBS and re-suspended in 1X binding buffer at a concentration of 1 × 106 cells/ml. Annexin V-FITC (5 μl) and propidium iodide (PI, 5 μl) were then added to 100 μl of cells. After being incubated for 15 min at room temperature in the dark, cells were immediately analysed by flow cytometry to identify early apoptotic cells (PI negative, FITC Annexin V positive).
HIF-1α immunolabelling in HEI-OC1 cells
HEI-OC1 cells were exposed to 0.8 mM H2O2 for 24 hours, washed twice with PBS and then fixed with 4% paraformaldehyde for 10 min. After washing again with PBS, cells were blocked with 1% bovine serum albumin (BSA) blocking solution for 30 min, incubated with anti-HIF-1α (1:1000, Novus, USA) in PBS/T at 4°C overnight. After washing with PBS/T, cells were incubated with a FITC-conjugated or TRITC-conjugated secondary antibody (1:1000, Invitrogen, USA) along with DAPI (1:800 dilution, Sigma-Aldrich, USA) in 0.1% Triton X-100 and 1% BSA in PBS at room temperature for 1 hour. Immunolabelling was observed under a confocal microscope (Leica, Germany).
HIF-1α and HDAC2 mRNA expression in PBMCs and HEI-OC1 cells
Total RNA was extracted from PBMCs and HEI-OC1 cells using TRIZOL Reagent (Invitrogen, USA). Total RNA (1 μg) was transcribed to cDNA using random hexamers and SuperScript reverse transcriptase. Expression of HIF-1α and HDAC2 were examined using the SYBR green Master Mix kit and Step One Plus™ Real-Time PCR System (Applied Biosystems, USA) 19. The sequences of primers used for RT-PCR are listed in Table I. The PCR conditions consisted of a pre-denaturation at 95°C for 4 min, 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 45 sec, and extension at 72°C for 1 min, and a final extension at 72°C for 10 min. Specificity of each PCR reaction was confirmed by melting curve analysis. HIF-1α and HDAC2 mRNA expression levels were calculated by the 2-(ΔΔCt) method.
HIF-1α and HDAC2 protein expression in PBMCs and HEI-OC1 cells
PBMCs and HEI-OC1 cells were harvested and lysed with RIPA buffer (KeyGEN, China) and a protease inhibitor cocktail (Sigma, USA) for 30 min at 4°C. Protein concentrations were calculated using the BCA Protein Assay Kit (Beyotime Biotechnology, China). A total of 20 μg of protein was denatured, separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were then blocked with 5% non-fat dried milk in Tris-buffered saline and Tween-20 (TBST) for 1 hour at room temperature, incubated with anti-HIF-1α (1:1000, Novus, USA), anti-HDAC2 (1:1000, CST, USA), or anti-β-actin (1:1000, CST, China) at 4°C overnight. Following three washes with TBST, the membranes were incubated with goat anti-mouse or goat anti-rabbit IgG antibody (1:2000, Abcam, UK) for 1 hour at room temperature. Finally, the immunoblots were detected using the Millipore ECL kit (Merck Millipore, USA). Density of each band was measured and quantified using Image J software ver. 1.34 (https://imagej.net/).
Statistical analysis
All statistical analyses were conducted using SPSS24.0 software. The data were expressed as mean ± SEM and analysed by unpaired Student t-test. A nonparametric (c2) test was used for categorical variables between groups. The Mann-Whitney rank-sum test was used when data were unevenly distributed. The correlation between HDAC2 and HIF-1α levels was analysed by Pearson correlation analysis. Statistical significance was set at p < 0.05.
Results
Clinical data
A total of 30 refractory SSNHL patients (14 males and 16 females) with a mean age of 43.4 ± 11.3 years were included in the study. The mean interval from onset to IMP was 17.7 ± 7.2 days. The demographic data is shown in Table II. There was no significant difference in PTA between the two groups before treatment (p > 0.05). After IMP, the overall efficacy was 53.3%, which is consistent with our previous reports 11,12. There was no significant difference in gender, age, side of ears affected, or number of patients with tinnitus and/or dizziness between the GCS and GCR groups (all p > 0.05). However, the GCR group had a significantly longer interval from onset to IMP than the GCS group (p < 0.05, Table II). Two months after IMP, significant hearing improvement was observed in 16 patients (the GCS group), while 14 patients had no hearing improvement (the GCR group). There was a significant difference in PTA between the two groups after treatment (p > 0.05).
Changes of HIF-1α and HDAC2 expression in PBMCs of SSNHL patients before and after IMP treatment
The expression of mRNA and protein levels of HIF-1α and HDAC2 were examined in PBMCs of 30 SSNHL patients and 10 normal controls. After IMP treatment, HIF-1α mRNA and protein levels were significantly decreased in the GCS group compared to the levels before treatment (p < 0.01), although HIF-1α levels were not changed in the GCR group (p > 0.05) (Fig. 2A,C). Additionally, the post-treatment expression of HIF-1α shows a significant increase in comparison with the control group. In contrast, following IMP treatment, HDAC2 mRNA and protein levels were significantly increased in the GCS group compared to the levels before treatment (p < 0.01), while HDAC2 protein levels were not significantly changed (p > 0.05) (Fig. 2B,D). These results confirm our previous results of HDAC2 down-regulation in patients with refractory SSNHL 11,12. The current results also suggest that HIF-1α may be involved in GC resistance in refractory SSNHL. HIF-1α levels in PBMCs might be used to predict response of SSNHL patients to GC treatment.
Apoptosis in HEI-OC1 cells exposed to H2O2 in vitro
To examine the relationship between HIF-1α and HDAC2 in refractory SSNHL, we firstly developed an in vitro model of OS by exposing HEI-OC1 cells to various concentrations of H2O2 (0 to 4 mmol/L). Cell viability and apoptosis were examined by flow cytometry. Significantly more apoptotic cells were observed in cells exposed to 0.8 mM or higher concentrations of H2O2 (all p < 0.01, Figure 3A,B). These results indicate that OS caused by 0.8 mM or higher concentrations of H2O2 induced significant apoptosis in HEI-OC1 cells. Significantly low viability was observed when the H2O2 concentration reached 1 mM and higher (Fig. 3D). The results shown in panels E and F indicate that the expression of HIF-1α and HDAC2 was increased and decreased separately with increasing concentrations of H2O2.
Effects of HIF-1α gene manipulation on activation of HDAC2 under OS and normal conditions
The RT-PCR and Western blot results described above suggest that HIF-1α may be involved in the down-regulation of HDAC2 in refractory SSNHL. To confirm this hypothesis, expression of HDAC2 was examined in HEI-OC1 cells when HIF-1α gene was silenced by siRNA or overexpressed by adenovirus under normal culture or OS (exposed to 0.8 mM of H2O2) conditions. Protein levels of HIF-1α and HDAC2 were evaluated by Western blot after HEI-OC1 cells had been transfected with HIF-1α siRNA or adenovirus under OS (Fig. 4). HIF-1α siRNA transfection significantly inhibited HIF-1α protein expression in normal culture condition (p < 0.05). Compared to the normal control group, OS significantly up-regulated HIF-1α expression (p < 0.05) and down-regulated HDAC2 expression (all p < 0.01). However, HIF-1α scRNA treatment reversed the changes induced by OS (all p > 0.05, Fig. 4G and H). HIF-1α over-expression induced by adenovirus transfection significantly up-regulated HIF-1α expression and down-regulated HDAC2 expression in normal culture condition (all p < 0.01). These changes induced by adenovirus were pronounced in cells exposed to OS (p < 0.01 or < 0.001, Fig. 4G,H).
Taken together, OS significantly up-regulated HIF-1α expression (p < 0.05) and down-regulated HDAC2 expression. However, these regulations were reversed to normal levels by HIF-1α scRNA. HDAC2 expression was dramatically inhibited in cells when HIF-1α was overexpressed, especially when cells were exposed to OS. These results confirm our hypothesis that HIF-1α negatively regulates HDAC2, especially under conditions of OS. Interestingly, silencing HIF-1α with scRNA could reverse the down-regulation of HDAC2 induced by OS.
Discussion
As first line-treatment, GCs are widely used to treat SSNHL 1. However, a significant number of patients are not responsive to the treatment and show GC resistance, the mechanisms of which are largely unknown. Progress in genetic pharmacologic research has revealed individual variations in GC resistance 23. A limited number of studies indicate that several molecular pathways that regulate HDAC2 are also involved in GC resistance 14,23. Decreased activity of HDAC2 contributes to steroid resistance in some diseases, such as chronic obstructive pulmonary disease and asthma 14,17,19. Our previous studies indicate that HDAC2 is associated with GC resistance in SSNHL, although the molecular mechanisms remain unclear 11-13. In the present study, increased expression of HIF-1α was observed in patients with refractory SSNHL and GC resistance accompanied by decreased expression of HDAC2. These results indicate that, besides HDAC2, HIF-1α is also involved with GC resistance in SSNHL. Furthermore, the levels of HIF-1α were decreased in PBMCs from patients who responded to IMP treatment, indicating that the levels of HIF-1α in PBMCs could be also used to predict prognosis of refractory SSNHL. We found that HDAC2 expression decreased when HIF-1α was overexpressed by adenovirus, although HDAC2 expression was not changed when HIF-1α expression was inhibited by siRNA in normal culture conditions. Interestingly, silencing HIF-1α with siRNA reversed HDAC2 down-regulation to normal levels in HEI-OC1 cells exposed to OS. These results indicate a close relationship between HIF-1α and HDAC2, especially under conditions of OS. Inhibition of HIF-1α could potentially up-regulate HDAC2 expression and reverse GC resistance to improve the effects of GC in refractory SSNHL patients.
To our knowledge, this is the first study to evaluate a possible relationship between HIF-1α and HDAC2 in cochlear cells under OS conditions. We believe that HIF-1α is an important factor that directly affects HDAC2 expression in HEI-OC1 cells under OS conditions. It can be speculated that OS in the cochlea up-regulates HIF-1α and then down-regulates HDAC2, inducing GC resistance in SSNHL patients. HDAC2 acts as a critical factor in GC resistance according to our previous studies and the literature 11-15. Knock-down of HDAC2 at the protein level enhanced the anti-inflammatory effects of GCs 24. HIF-1α may be one of the signals released by the cochlea in the early phases of OS in response to damage caused by increased levels of ROS 25. Refractory SSNHL patients who did not respond to IMP treatment had a significantly higher expression of HIF-1α and lower expression of HDAC2 than those who responded to the treatment. It is clear that the HIF-1α/HDAC2 pathway is involved in GC resistance in patients with refractory SSNHL 14,15. Therefore, the HIF-1α/HDAC2 pathway may provide a target to treat GC resistance in SSNHL patients in the future. For example, silencing HIF-1α with siRNA could have potential to treat SSNHL when patients do not respond to GCs.
Previous studies indicate that OS is involved in the pathogenesis of SSNHL and GC resistance 4-7. However, the mechanisms of OS in the pathogenesis of SSNHL and GC resistance are still unclear. Based on our in vitro OS model, OS up-regulates the expression of HIF-1α and inhibits the expression of HDAC2 in HEI-OC1 cells. In refractory SSNHL patients, intensive OS could cause up-regulation of HIF-1α and down-regulation of HDAC2 in the cochlea, leading to GC resistance. We believe this is why a combination of an antioxidant and GC is more effective than GC alone to treat SSNHL 5,7-9. In addition, all patients in the present study failed systemic GC treatment before IMP. After IMP plus antioxidant treatment, significant hearing improvement was found in 53% of patients, indicating that IMP plus antioxidant is an effective treatment for refractory SSNHL.
Conclusions
In summary, our results suggest that both HIF-1α and HDAC2 are involved in GC resistance in refractory SSNHL. HIF-1α inhibition is able to up-regulate HDAC2, and relieves GC resistance prospectively. HIF-1α could be a potential biomarker to predict the prognosis of refractory SSNHL and a possible target for the treatment of SSNHL in the future.
Acknowledgements
We would like to give our sincere thanks to Drs. Xiaoping Du and Zachary Yokell (Hough Ear Institute, Oklahoma, USA) for their critical review and thoughtful feedback during the preparation of this manuscript.
Conflict of interest statement
All authors declare no conflict of interest.
Funding
Natural Science Foundation of China (81670931); Medical Science and Technology Development Foundation of Nanjing Municipality Health Bureau (ZKX21012); Youth Science and Technology Project from Suzhou Health Commission, Jiangsu, China (KJXW2021064); Natural Science Foundation of Jiangsu, China (BK20231122)
Author contributions
WS: designed the study concept, gave administrative support and critically revised the manuscript for important intellectual content; ZG, WS: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; ZG: wrote and revised the whole manuscript; WZ, ZG, JH: collected clinical data separately; WM, XC, MG: analysis and interpret the data. All authors read and commented on the final draft, agreed to be accountable for all aspects of the work.
Data availability statement
The raw data supporting the conclusions of this article will made available by the authors, without undue reservation.
Ethical consideration
The studies involving human participants were reviewed and approved by ethics committee of Nanjing Drum Tower Hospital (IRB2016-194-01).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: March 22, 2024
Accepted: July 2, 2024
Figures and tables
Figure 1. Construction of adenovirus expressing GV314-CMV-Flag-GFP. (A) adenovirus expressing GV314-CMV-Flag-GFP and (B) recombinant HIF-1α.
Figure 2. HIF-1α/HDAC2 mRNA and protein levels in refractory SSNHL patients following therapy compared with the control group. The post-treatment expression of HIF-1α mRNA and protein significantly decreased compared with the pre-treatment expression in the GCS group (**p < 0.01). No significant change was observed between pre- and post-treatment results in GCR group, while a large significant difference is observed in the post-treatment and control groups (**p < 0.01). Similarly, the expression of HIF-1α protein in the GCS group significantly decreased following therapy (**p < 0.01), but there is no obvious difference between pre- and post-treatment in GCR group. A significant difference was seen in the post-treatment and control groups (**p < 0.01). Expression of HDAC2 mRNA and protein levels was significantly higher in the post-treatment of GCS group (**p < 0.01), while no significant difference was observed in the GCR group (p > 0.05).
Figure 3. Comparison of various concentrations of H2O2 on apoptosis in HEI-OC1 cells. All control groups were HEI-OC1 cells without H2O2 treatment. Representative images of HEI-OC1 cells treated with H2O2 at final concentrations of 0.5, 0.8, 1, 2 and 4 mM for 24 hours and stained with FITC Annexin V. More apoptotic cells were observed as the concentration of H2O2 was increased (A). Percentage of apoptotic cells in HEI-OC1 cells exposed to different concentrations of H2O2. Significantly more apoptotic cells were observed in the cells exposed to high concentrations of H2O2 (> 1 mM) compared to the control group (B and C, **p < 0.01). Comparison of cell viability of HEI-OC1 cells treated with different concentrations of H2O2 (D). Representative images and statistical analyses of Western blotting of HIF-1α and HDAC2 in HEI-OC1 cells exposed to various concentrations of H2O2. (E and F).
Figure 4. HDAC2 protein expression in HEI-OC1 cells after the HIF-1α gene was silenced or overexpressed. (A-F). Fluorescence images of cells transfected with HIF-1α siRNA (A-C) or HIF-1α overexpression adenovirus (D-F). Cells were immunolabelled with HIF-1α (red, A and D) and stained with DAPI (blue in C and F). Green fluorescence in B and E indicates successful transfection of HIF-1α siRNA (FAM, B) or HIF-1α adenovirus (GFP, E). Weak HIF-1α staining was observed in cells treated with HIF-1α siRNA (A), while strong HIF-1α staining was observed in cells treated with HIF-1α adenovirus (Ad-HIF-1α, D). Representative images of Western blotting of HIF-1α and HDAC2 in HEI-OC1 cells transfected with HIF-1α siRNA or adenovirus under normal culture or OS conditions (G). Effects of HIF-1α silencing or overexpression on HDAC2 and HIF-1α protein expression in HEI-OC1 cells (H). HIF-1α siRNA significantly inhibited HIF-1α protein expression in normal culture conditions (p < 0.05). OS significantly increased the HIF-1α-protein level (p < 0.05) and decreased HDAC2 protein levels (p < 0.01). However, HIF-1α siRNA treatment reversed these changes induced by OS (all p > 0.05 compared to normal controls). HIF-1α adenovirus significantly increased HIF-1α protein level and decreased HDAC2 protein level in the normal culture condition (p < 0.01 or 0.001). The effects of HIF-1α-overexpression were pronounced in cells exposed to OS p < 0.01). n.s indicates not significant difference, *, ** and *** indicate p < 0.05, < 0.01 and < 0.001, respectively, compared to untreated cells.
| mHDAC2-realtime-F: | 5’-ATGGCGTACAGTCAAGGAGG-3’, |
| mHDAC2-realtime-R: | 5’-ATGAGGCTTCATGGGATGACC-3’, |
| mHIF-1α-realtime-F: | 5’-CTCAGCCCCAGTGCATTGTA-3’, |
| mHIF-1α-realtime-R: | 5’-GAACCTCCTATAGCCACCGC-3’, |
| mVEGF-realtime-F: | 5’-TCGGGCCTCCGAAACCATGA-3’, |
| mVEGF-realtime-R: | 5’-CCTGGTGAGAGATCTGGTTC-3’, |
| mβ-actin-realtime-F: | 5’-GGCTGTATTCCCCTCCATCG-3’, |
| GCS group (n = 16) | GCR group (n = 14) | p-value | |
|---|---|---|---|
| Gender (M:F) | 10:6 | 4:10 | 0.06a |
| Mean age (years, mean ± 95% CI) | 41.2 ± 9.6 | 45.8 ± 13 | 0.28b |
| Number of ears affected (L:R) | 9:7 | 7:7 | 0.73a |
| Number of patients with tinnitus (%) | 15/16 (93.7%) | 12/14 (85.7%) | 0.46a |
| Number of patients with dizziness (%) | 7/16 (43.7%) | 9/14 (64.2%) | 0.26a |
| Interval from onset to IMP (days, median ± IQR) | 13.3 ± 3.8 | 22.6 ± 8.1 | < 0.05c |
| PTA before treatment (dB, mean ± 95% CI) | 105.5 ± 18.6 | 101.1 ± 14.9 | > 0.05b |
| PTA after treatment (dB, mean ± 95% CI) | 65.1 ± 22.7 | 98.4 ± 18.8 | < 0.05b |
| a: Chi-squared test; b: independent-sample t-test; c: rank-sum test. | |||
References
- Chandrasekhar S, Do B, Schwartz S. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. 2019;161:S1-S45. doi:https://doi.org/10.1177/0194599819859885
- Ungar O, Cavel O, Oron Y. A subjective rating scale for initial assessment of sudden unilateral sensorineural hearing loss. Audiol Neurotol. 2017;22:154-159. doi:https://doi.org/10.1159/000479723
- Schilder A, Su M, Blackshaw H. Hearing protection, restoration, and regeneration: an overview of emerging therapeutics for inner ear and central hearing disorders. Otol Neurotol. 2019;40:559-570. doi:https://doi.org/10.1097/MAO.0000000000002194
- Becatti M, Marcucci R, Mannucci A. Erythrocyte membrane fluidity alterations in sudden sensorineural hearing loss patients: the role of oxidative stress. Thromb Haemost. 2017;117:2334-2345. doi:https://doi.org/10.1160/TH17-05-0356
- Pool C, King T, Pradhan S. Sudden sensorineural hearing loss and coronavirus disease. J Laryngol Otol. 2022;136:823-826. doi:https://doi.org/10.1017/S0022215122000068
- Elias T, Monsanto R, Amaral J. Evaluation of oxidative-stress pathway and recovery of sudden sensorineural hearing loss. Int Arch Otorhinolaryngol. 2021;25:428-432. doi:https://doi.org/10.1055/S-0040-1714130
- Bai X, Chen S, Xu K. N-acetylcysteine combined with dexamethasone treatment improves sudden sensorineural hearing loss and attenuates hair cell death caused by ROS stress. Front Cell Dev Biol. 2021;9. doi:https://doi.org/10.3389/fcell.2021.659486
- Paprocki J, Sutkowy P, Piechocki J. Association between Vitamin D supplements, oxidative stress biomarkers, and hyperbaric therapy in patients with sudden sensorineural hearing loss. Oxid Med Cell Longev. 2021;2021. doi:https://doi.org/10.1155/2021/8895323
- Murray D, Fagan P, Ryugo D. Idiopathic sudden sensorineural hearing loss: a critique on corticosteroid therapy. Hear Res. 2022;422. doi:https://doi.org/10.1016/j.heares.2022.108565
- Nakagawa T, Kumakawa K, Usami S. A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 2014;12. doi:https://doi.org/10.1186/s12916-014-0219-x
- Hou J, She W, Du X. Histone deacetylase 2 in sudden sensorineural hearing loss patients in response to intratympanic methylprednisolone perfusion. Otolaryngol Head Neck Surg. 2016;154:164-170. doi:https://doi.org/10.1177/0194599815606911
- Zhang X, Chen J, Gao Z. Response of glucocorticoid receptor alpha and histone deacetylase 2 to glucocorticoid treatment predicts the prognosis of sudden sensorineural hearing loss. Clin Exp Otorhinolaryngol. 2019;12:367-375. doi:https://doi.org/10.21053/ceo.2018.01298
- Qi H, Gao Z, Hou J. Nuclear factor erythroid 2-related factor 2-histone deacetylase 2 pathway in the pathogenesis of refractory sudden sensorineural hearing loss and glucocorticoid resistance. ORL J Otorhinolaryngol Relat Spec. 2021;83:227-233. doi:https://doi.org/10.1159/000515205
- Barnes P. Glucocorticosteroids. Handb Exp Pharmacol. 2017;237:93-115. doi:https://doi.org/10.1007/164_2016_62
- Yang N, Ray D, Matthews L. Current concepts in glucocorticoid resistance. Steroids. 2012;77:1041-1049. doi:https://doi.org/10.1016/j.steroids.2012.05.007
- Yang C, Zhong Z, Wang S. HIF-1: structure, biology and natural modulators. Chin J Nat Med. 2021;19:521-527. doi:https://doi.org/10.1016/S1875-5364(21)60051-1
- Liu Q, Hua L, Bao C. Inhibition of spleen tyrosine kinase restores glucocorticoid sensitivity to improve steroid-resistant asthma. Front Pharmacol. 2022;13. doi:https://doi.org/10.3389/fphar.2022.885053
- Bergström A, Fog K, Sager T. Competitive HIF prolyl hydroxylase inhibitors show protection against oxidative stress by a mechanism partially dependent on glycolysis. ISRN Neurosci. 2013;2013. doi:https://doi.org/10.1155/2013/598587
- Chikuma K, Arima K, Asaba Y. The potential of lipid-polymer nanoparticles as epigenetic and ROS control approaches for COPD. Free Radic Res. 2020;54:829-840. doi:https://doi.org/10.1080/10715762.2019.1696965
- Guideline of diagnosis and treatment of sudden deafness (2015). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;50:443-447. doi:https://doi.org/10.3760/cma.j.issn.1673-0860.2015.06.002
- Bett A, Haddara W, Prevec L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802-8806. doi:https://doi.org/10.1073/pnas.91.19.8802
- Addison C, Hitt M, Kunsken D. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J Gen Virol. 1997;78:1653-1660. doi:https://doi.org/10.1099/0022-1317-78-7-1653
- Iudicibus S, Franca R, Martelossi S. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol. 2011;17:1095-1108. doi:https://doi.org/10.3748/wjg.v17.i9.1095
- Li J, Liu D, Wu J. Ginsenoside Rg1 attenuates ultraviolet B-induced glucocortisides resistance in keratinocytes via Nrf2/HDAC2 signalling. Sci Rep. 2016;6:1-11. doi:https://doi.org/10.1038/srep39336
- Guan D, Su Y, Li Y. Tetramethylpyrazine inhibits CoCl2-induced neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression of HIF1α/NOX2/ROS pathways. J Neurochem. 2015;134:551-565. doi:https://doi.org/10.1111/jnc.13161
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2024 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 495 times
- PDF downloaded - 230 times




 PDF
PDF
