Audiology
Vol. 44: Issue 5 - October 2024
Long-term safety and subjective satisfaction of Bonebridge and Vibrant Soundbridge in congenital unilateral conductive hearing loss
Abstract
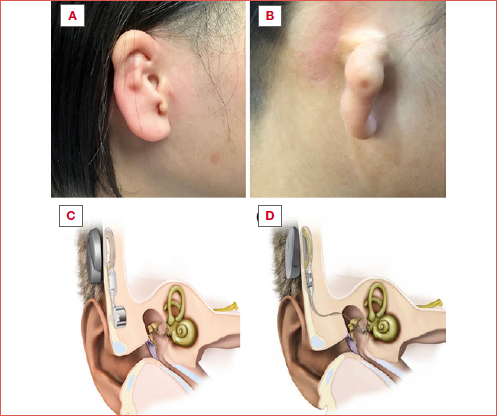
Purpose. The objective of this study was to assess and compare the long-term safety and subjective satisfaction levels of Bonebridge (BB) and Vibrant Soundbridge (VSB) in patients with congenital unilateral conductive hearing loss (UCHL).
Methods. The hearing effectiveness was measured using sound field hearing threshold (SFHT) and word recognition score (WRS). Long-term safety and subjective satisfaction levels were measured by questionnaires, including Speech, Spatial, and Qualities of Hearing Scale-12 (SSQ-12) and Abbreviated Profile of Hearing Aid Benefit (APHAB).
Results. Both BB and VSB were effective in improving the hearing of patients with congenital UCHL. Four of the 20 patients discontinued wearing the device postoperatively, and no complications were reported by long-term users. Patients experienced an overall improvement in their hearing experience across most subscales of SSQ-12 and a lower hearing problem rate across most subscales of the APHAB after implantation. However, the ‘quality of hearing’ subscale in the SSQ-12 did not show any improvement, and the aversiveness subscale in the APHAB exhibited a relatively higher percentage of reported problems after BB or VSB implantation. No significant differences were observed in SFHT, WRS, SSQ-12, and APHAB between the two groups.
Conclusions. Both BB and VSB are effective in improving the hearing of UCHL patients, with high subjective satisfaction and low complication rates in the long-term postoperative period, with no significant difference between the two devices. Follow-up device fitting is essential, especially to address increased device sound or noise after surgery, reduce non-usage rates and achieve higher subjective satisfaction levels.
Introduction
Unilateral conductive hearing loss (UCHL) can have a significant impact on patients, who are at a higher risk of experiencing delayed speech development, poor academic performance, reduced quality of life, and depression compared to individuals with normal hearing (NH) 1. Several hearing rehabilitation interventions, including conventional hearing aids (HAs), bone conduction hearing aids (BCHAs), bone conduction implants (BCIs), and active middle ear implants (AMIs), have demonstrated significant improvements in audiological outcomes for patients with UCHL 2,3. These findings underscore the importance and necessity of hearing intervention for patients with UCHL. Microtia and atresia (MA) is a congenital malformation of the external and middle ear, characterised by the absence or stenosis of the external auditory canal, as well as varying degrees of middle ear malformation. The incidence of MA is approximately 1 in 10,000 live births, with rates varying by ethnic background 4. About three-quarters of cases of MA present unilateral malformations. In most patients with unilateral MA (UMA), the outer and middle ear structures are affected, while the inner ear functions normally, making it a typical form of UCHL.
Canaloplasty has been a common surgical option for restoring hearing in patients with UMA 5,6; however, it is only suitable for a limited number of potential candidates. Typically, only patients with a Jahrsdoerfer score greater than 7 and aged over 6 years are expected to achieve adequate postoperative hearing, and even then long-term stable hearing improvement is unsatisfactory 7,8. Additionally, the outer ear deformity in these patients often makes them unsuitable candidates for conventional air conduction HAs. Therefore, non-surgical options such as BCHA devices are viable solutions to manage hearing loss in patients with UMA 3,9. BCHAs improve hearing on the affected side by transmitting sound through an audio processor (AP) attached to the skull with a soft band, headband, or spectacles through bone conduction stimulation. However, factors such as limited percutaneous hearing gain, complications related to prolonged local skin compression, and unattractive appearance can reduce patient compliance, especially in older patients who have more self-perception 10.
Thus, hearing implants, such as the BCI and AMI, are utilised in patients with UMA to achieve a more stable and reliable improvement in hearing and subjective satisfaction 2,11. The Bonebridge (BB, Med-EL; Innsbruck, Austria) is an active percutaneous BCI and is a viable option for patients with UMA over 5 years of age who have conductive or mixed hearing loss with bone conduction thresholds of 45 dB HL or less at 0.5, 1, 2, and 4 kHz 12. The Vibrant Soundbridge (VSB, Med-El, Innsbruck, Austria), a direct-drive, partly implantable middle ear hearing device can be implanted in patients with UMA over 3 years of age who have conductive or mixed hearing loss with their bone conduction (BC) thresholds at or not worse than 65 dB HL across frequencies of 0.5-4 kHz 13. Although previous studies have investigated the hearing benefits of BB and VSB in patients with UMA, limited research has focused on the long-term subjective evaluation of the quality of life in this population after implantation.
The current study had two objectives: (1) to evaluate the long-term safety and subjective satisfaction levels of BB and VSB in patients with congenital UCHL and (2) to determine if there were any differences in self-reported satisfaction between patients implanted with a BB or a VSB.
Materials and methods
Patients
Between April 2015 and March 2022, a total of 20 patients with UMA who were implanted with either a VSB or BB were recruited from a single centre at the Department of Otolaryngology, Head and Neck Surgery, Capital Medical University affiliated Beijing Tongren Hospital. The mean age at implantation was 11.5 ± 7.8 years. The VSB was recommended for patients with a Jahrsdoerfer score of 7 or higher and without any contraindications for clip coupler implantation of the stapes head (such as stapes footplate fixation and tympanic cavity stenosis). For patients who did not meet these criteria, the BB was recommended. The final decision on the implant type was made by the patients and their parents. Demographic data, results of pure-tone average (PTA) between 0.25-4 kHz, device type, and surgery records were obtained from hospital medical charts and are shown in Table I.
The study included patients who met the following criteria: 1) diagnosed with UMA with no other combined congenital disorders; 2) age ≥ 5 years old; 3) PTA BC between 0.25 and 4 kHz of the impaired ear ≤ 45 dB HL with an air-bone conduction gap (ABG) ≥ 20 dB HL; 4) PTA air-conduction (AC) between 0.25 and 4 kHz of contralateral ear ≤ 20 dB HL without ABG; 5) use of Mandarin as native speech-language; 6) cooperate well with telephone or outpatient follow-up after implantation surgery.
Surgical procedure
Patients who had previously undergone total auricular reconstruction underwent BB or VSB implantation alone. Those who had not yet received auricular reconstruction were treated with a combined approach, involving two or three-stage surgical auricular reconstruction and hearing implantation, as described in our previous studies 14-16. The selection of the auricular reconstruction method was contingent upon the condition of the skin posterior to the ear and the patient’s preference. The implantation of VSB or BB was integrated into the final stage of the auricular reconstruction.
Audiological evaluation
The BB and VSB were activated and fitted with the Symfit BAO software by a senior audiologist after wound healing and subsidence of skin flap swelling on the surgical site. The device settings during the postoperative evaluation are uniformly omnidirectional microphones, with volume settings determined by patient preference. For patients implanted with the BB, the fitting was performed at least 1 week after surgery, while for VSB users was at least six weeks after surgery.
Sound field hearing threshold (SFHT) and speech perception were performed preoperatively and postoperatively in a sound-attenuated audiometric booth with the loudspeakers positioned 1 m from the centre of the participant’s head. All postoperative audiological tests were conducted after the initial device fitting. Warble tones were used to determine the hearing thresholds (in dB HL) and the SFHT represented the average of five frequencies of 0.25, 0.5, 1, 2, and 4 kHz. Speech perception was evaluated using the Mandarin Speech Test Materials (MSTM), which employed disyllabic word lists. The word recognition score (WRS) was quantified as a percentage and assessed in a quiet environment. During this assessment, the healthy ear was occluded using both an earmuff and an earplug inserted into the external auditory canal. Additionally, the speech reception threshold (SRT, in dB SNR) was measured in a noise background fixed at 65 dB SPL, with both ears left uncovered. Both the speech and noise stimuli were presented from directly ahead (0° azimuth, S0N0 configuration). The speech level was incrementally adjusted by 2 dB steps based on the accuracy of word recognition. The SRT, defined as the hearing level at which 50% of words are correctly repeated, was calculated to be minus 65 dB SPL.
Long-term follow-up
All participants underwent comprehensive long-term follow-up lasting a minimum of 14 months. Patients and their guardians are in continuous communication with the evaluators, who executed follow-ups on a quarterly basis by telephone. These follow-ups assess several criteria, including the utilisation of the device, any local skin issues, and the auditory stability afforded by the device. Additionally, subjective satisfaction was evaluated through questionnaires distributed via email to either the patients or their guardians. The comparative hearing condition before and after hearing implantation was evaluated using questionnaires of the Speech, Spatial, and Qualities of Hearing Scale -12 (SSQ-12), a shortened version of the traditional SSQ consisting of 12 questions 17 and the Abbreviated Profile of Hearing Aid Benefit (APHAB) 18. The SSQ-12 assesses the subject’s ability to hear speech in 3 subscales of ‘speech hearing’, ‘spatial hearing’, and ‘qualities of hearing’. The APHAB includes subscales of aversiveness to sound (AV), hearing in background noise situations (BN), ease of communication (EC), and hearing in a reverberant setting (RV). Subjective satisfaction benefits are represented by ‘delta’, which is the difference between the scores of each sub-item in the questionnaire after hearing implantation and the scores before surgery. For patients younger than 12 years, the questionnaire was to be filled out with parental assistance, whereas patients aged 12 years and older could complete the questionnaire independently.
Data analysis
Results were presented as means ± standard deviations (SD). All analyses used two-tailed tests, with statistical significance set at p < 0.05. The Wilcoxon signed-rank test or paired-t test was applied to compare pre- and postoperative audiological outcomes. The Mann-Whitney U test or independent-t test was applied to compare audiological outcomes between the VSB and BB groups. Statistical analyses were conducted using IBM SPSS Statistics (IBM, Armonk, New York) and graphs were created using GraphPad Prism 8 (GraphPad Software, Inc.).
Results
Demographics, audiological characteristics, and surgical procedure
Table I presents the demographics and audiological characteristics of 20 patients, with 10 who were fitted with a BB and 10 fitted with a VSB. The mean age at implantation was 10.3 ± 7.2 years for the BB and 12.7 ± 8.6 years for the VSB group, and there was no significant difference in age at implantation between the two groups (p = 0.51). All patients had UCHL with normal hearing (NH) in the contralateral ear. Among BB users, 8 patients had atresia and 2 had stenosis. The PTA of 0.25-4 kHz was 73.7 ± 7.3 dB HL for AC and 12.8 ± 4.8 dB HL for BC. For VSB users, all 10 patients had atresia. The PTA threshold of 0.25-4 kHz was 70.5 ± 10.1 dB HL for AC and 10.3 ± 8.1 dB HL for BC. There were no significant differences in PTA AC (p = 0.43) and PTA BC (p = 0.41) between the two groups.
In the BB group, eight patients underwent a two-stage auricular reconstruction procedure combined with BB implantation, the mean duration of which was 163.5 ± 8.8 min. Additionally, one patient opted for a three-stage auricular reconstruction combined with BB implantation, with surgical time of 180 min., and one patient had an isolated BB implantation the duration of which was 45 min. In the VSB group, nine patients underwent the two-stage auricular reconstruction along with VSB implantation, with a mean surgical time of 201.6 ± 9.4 min. and two patients underwent a standalone VSB implantation, with a mean surgical time of 67.5 min. A comparative analysis of the combined hearing implantation and two-stage auricular reconstruction procedures revealed that the VSB group had a significantly longer surgical time than the BB group (VSB vs BB: 201.6 ± 9.4 vs 163.5 ± 8.8 min., p < 0.01).
Sound field hearing thresholds and speech perception
In the BB group, the average preoperative SFHT was 57.9 ± 3.7 dB HL, which significantly decreased (became better) to 35.5 ± 3.8 dB HL in the postoperative aided condition (p < 0.01, Figure 1A), resulting in a mean functional hearing gain (FHG) of 22.4 ± 3.5 dB HL. In the VSB group, patients exhibited a mean preoperative SFHT of 58.9 ± 8.2 dB HL, which significantly decreased (became better) to 35.7 ± 3.9 dB HL in the postoperative aided condition (p < 0.01, Figure 1A) with a FHG of 23.2 ± 6.6 dB HL. The mean WRS and SRT obtained from the MSTMs are presented in Figures 1B-C. In the unaided condition, the BB group had a mean score of 20.1 ± 10.5%, which significantly improved to 84.8 ± 2.7% with an improvement of 67.4 ± 10.5% (p < 0.01). Similarly, the VSB group had a mean score of 22.1 ± 4.7% in the unaided condition, which significantly improved to 81.6 ± 5.3% with an improvement of 59.5 ± 9.3% (p < 0.01). In the unaided condition, the average SRT for the BB group was -2.1 ± 1.8 dB SNR. This value significantly decreased (became better) to -5.3 ± 0.9 dB SNR, representing a decrement of 3.2 ± 1.5 dB SNR (p < 0.01). Similarly, in the unaided condition, the VSB group exhibited a mean SRT of -3.6 ± 1.9 dB SNR. Post-implantation, this value significantly decreased (became better) to -5.6 ± 0.5 dB SNR, indicating a decrement of 2 ± 2 dB SNR (p < 0.05). Furthermore, no statistically significant differences were observed in the SFHT, FHG, WRS, and SRT between patients in the BB and VSB groups.
Device use and compliance
During follow-up via email, all patients reported experiencing stable hearing from the device in the impaired ear, and none reported complications related to device failure or surgery. Of these, 16 reported consistent daily use of the device during the postoperative period, ranging from 4 to 14 hours per day (the average daily usage time was 6.8 ± 3.2 hours for the BB and 8.1 ± 3 hours for the VSB group). Four patients did not use the device (3 from the BB group and 1 from the VSB group), and the reasons for their non-usage were investigated. Patient 2, a 35-year-old male, had difficulty adapting to the amplified sound in the affected ear, possibly due to long-term unilateral hearing deprivation. Patient 5, an 8-year-old male, and Patient 18, a 12-year-old female, reported hearing background noise overly amplified by the device. Unfortunately, they were unable to come to the hospital for a device fitting due to scheduling conflicts. Patient 9, a 5-year-old male, was not fitted with the device postoperatively due to concerns about potential detachment during playtime with peers. Consequently, his parents chose to defer device fitting until the child is older.
Long-term subjective satisfaction
According to the SSQ questionnaire, 15 of 16 patients exhibited an improvement in their overall hearing experience (indicated by a positive delta overall score) following the implantation, with only 1 patient (P15, implanted with a VSB) reporting no change (delta overall score of 0) on the SSQ questionnaire (Fig. 2). Prior to BB implantation, the mean scores for subscales of ‘speech discrimination’, ‘spatial hearing’, and ‘overall’ were 6.7 ± 1.5, 4.5 ± 2.5, and 6.2 ± 1.1, respectively. After implantation, these scores significantly improved to 9.4 ± 0.8, 8.7 ± 1.3, and 8.6 ± 1.2, respectively (all p < 0.05, Figure 3A). However, there was no significant difference in the ‘qualities of hearing’ between unaided (score: 6.8 ± 1.2) and aided (score: 7.6 ± 1.8) conditions (p = 0.24, Figure 3A). Similarly, following VSB implantation, significant improvements were also observed in the subscale scores of ‘speech discrimination’ (7.7 ± 1.6 vs 9.1 ± 0.8), ‘spatial hearing’ (5.4 ± 2.9 vs 7.7 ± 2.5), and ‘overall’ (7 ± 1.7 vs 8.2 ± 1.3), with p < 0.05 (Fig. 3B). No significant difference was found in the ‘qualities of hearing’ subscale score between the unaided (score: 7.2 ± 1.6) and aided (score: 7.5 ± 1.5) conditions (p = 0.24, Figure 3B).
According to the APHAB questionnaire, 10 of 16 patients reported a decrease in their problems in the BN, EC, and RV dimensions, while 12 of 16 patients reported an increase in their problems in the AV dimension compared to before implantation. There was no difference observed in the P1, P2, and P3 dimensions before and after implantation (Fig. 4). In the BB group (Fig. 5A), the mean problem percentage of APAHB decreased markedly with the hearing implants, with particularly significant improvements in the mean scores of subscales in BN (unaided vs aided, 34.2 ± 24.7% vs 24 ± 19.3%, p < 0.05), EC (unaided vs aided, 12.1 ± 12.9% vs 3. ± 4.4%, p < 0.05), and RV (unaided vs aided, 34.4 ± 23.6% vs 27.1 ± 20.3%, p < 0.05). However, a significantly increased problem rate was found in the subscale of AV with a mean score of 11.5 ± 14.5% in the unaided condition and 35.8 ± 26.1% after the implantation (p < 0.05, Figure 5A). The VSB group exhibited a marked reduction (better) in the mean problem percentage of APAHB following the implantation (Fig. 5B), with a notable decrease observed in the mean scores of the BN, EC, and RV subscales (unaided vs aided, 45.5 ± 18.1% vs 35.3 ± 14.7%, p < 0.05; 9.9 ± 12.1% vs 3.6 ± 3.4%, p < 0.05; 46.1 ± 21.2% vs 38.8 ± 16.3%, p < 0.05, respectively). In contrast, a significantly increased problem rate was observed in the AV subscale, with mean scores of 22.4 ± 20.6% in the unaided condition and 43.7 ±25.2% after implantation (p < 0.01, Figure 5B).
Moreover, the study did not reveal any significant differences in the SSQ and APHAB scores between patients who received the implantation of BB or VSB.
Discussion
Conventional BCHAs typically require softbands, headbands, spectacles, or similar attachments to secure the AP to the mastoid and apply static pressure on the skin to transmit bone-conducted vibrations efficiently to the cochlea. However, the use of such coupling methods may diminish patient acceptance because of their noticeable appearance and discomfort during use. Moreover, the placement of the AP on the skin surface leads to reduced effectiveness of the bone conduction signal due to the dampening effect of the skin and subcutaneous soft tissue, resulting in suboptimal hearing aid outcomes compared to BB and VSB, which directly vibrate the skull or ossicles 10. Previous studies have demonstrated the audiological efficacy of hearing implants in patients with congenital UCHL 2,3. The present study aimed to investigate the long-term use, safety, and subjective satisfaction of two common hearing implants (BB and VSB) in patients with congenital UCHL, and to determine if there are any differences in hearing outcomes between the two devices.
Audiological benefits
Initially, we evaluated the audiological effects of BB and VSB in our cohort of patients by conducting a full hearing assessment, including the FHG across 0.25-4 kHz of hearing threshold, WRS and SRT of speech perception tests. The mean FHG for the BB and VSB groups were 22.4 ± 3.5 dB HL and 23.2 ± 6.6 dB HL, respectively. Additionally, the mean improvement in WRS was 67.4 ± 10.6% and 59.5 ± 9.3% for the BB and VSB groups, respectively, and these hearing outcomes are consistent with previously reported data regarding the application of BB and VSB in patients with UCHL or CHL 19-21. Theoretically, BCDs may introduce disruptive auditory inputs to the normally hearing ear. This effect, known as cross hearing 22, is primarily attributed to the limited transcranial attenuation of bone-conducted sound. In our study, the SRT in the aided condition was -5.3 ± 0.9 dB SNR for the BB group and -5.6 ± 0.5 dB SNR for the VSB group. A comparative analysis of SRT between these groups revealed no significant differences, suggesting that the cross-hearing effect of BCDs does not significantly impair bilateral hearing abilities compared to VSB. This aligns with another study comparing BB and VSB in sound localisation for UCHL patients, where localisation performance with BB, despite cross-hearing, was comparable to that with VSB 2. Additionally, the literature on the use of VSB and BB in congenital UCHL presents diverse outcomes, and some studies report notable improvements in speech perception and spatial hearing among congenital UCHL patients using BCD or VSB. In contrast, other research indicates that while there may be benefits in speech perception, spatial hearing does not show marked improvement in congenital UCHL 2,21. However, other studies indicate benefits in speech perception but not in spatial hearing for congenital UCHL 23,24. This suggests that individuals with congenital UCHL might have adapted to their auditory limitations, relying on spectral shape cues and the monaural head shadow effect that developed during prolonged unilateral hearing deprivation. Consequently, the introduction of a BCD or VSB could disrupt these established auditory cues, potentially compromising innate directional hearing abilities 25.
Compliance and complication
A long-term follow-up evaluation was conducted, and 4 patients did not continue to wear the hearing implant postoperatively, with 3 patients from the BB group and 1 from the VSB group. The 5-year-old patient was not fitted with the device postoperatively due to parental concerns about potential detachment during playtime with peers. Three patients who stopped using the device reported amplified sound or noise and difficulty adapting to binaural hearing. Sixteen patients consistently used the device during the postoperative period, with an average daily usage time of 6.8 ± 3.2 hours for the BB and 8.1 ± 3 hours for the VSB. Although it was not possible to compare the daily usage time with BCHAs as none of the enrolled patients used them, the results of this study align with prior research on the use of VSB in preschool children with congenital UAA, which reported a markedly longer daily usage time for VSB with an average of 10 hours per day compared to 2 hours per day for BCHAs 26.
Moreover, our study yielded encouraging results regarding the safety of BB and VSB in patients with congenital UCHL with no complications related to the device failure or implantation. In 2011, the first BB implantation was carried out in Austria 12. Subsequently, several published articles reported complications related to the device or surgery, such as minor skin infections that require only oral antibiotics and deeper infections that necessitate the removal of the implanted device 27,28. However, the overall incidence of these complications was low. A systematic review of outcomes in patients with BB reported that reoperation was rare, with only 1 of 117 patients requiring surgery for revision 12. The VSB was initially introduced for use in patients with mild to severe SNHL in the late 1990s 29. A review in 2016 evaluated the safety of VSB to treat SNHL and found that adverse events with VSB implantation were generally low and the overall failure rate was only 2.6%, mainly due to implant failure (36.8%), problems with floating mass transducer fixation during surgery, or postoperative dislocation due to MRI or fibrous tissue (18.4%) 30. In a study by Cadre et al. 11 which included 18 patients with CAA who were followed for 6.5 years, no cases of facial palsy, skin damage, revision surgery, or dizziness were reported.
Long-term subjective satisfaction
Subjective satisfaction questionnaires of SSQ-12 and APHAB were distributed to patients who continued to use the devices after a minimum of 14 months (mean follow-up time: 36.7 ± 18.1 months) postoperatively. In the SSQ questionnaire, most patients (15/16) reported improved overall hearing experience after implantation, with mean scores of 8.6 ± 1.2 and 8.2 ± 1.3 for the BB and VSB, respectively (Fig. 2). Consistent with our findings, a study by Hirth et al. 9 reported an overall mean hearing score of 7.8 ± 1 with the use of an adhesive BCHA in patients with UCHL. Volgger et al. 21 found that 80% of UCHL patients reported an improvement in overall hearing experience after BB implantation based on a post-implantation subjective benefit assessment using a version of SSQ with 49 questions. However, there is a lack of literature regarding SSQ outcomes with VSB in individuals with congenital UCHL. Patients with UCHL exhibit compromised binaural auditory functions, which detrimentally affects their abilities in speech recognition in noise and sound localisation. Regarding speech perception, consensus exists that individuals with UCHL can derive benefit from BCD and VSB 20,21. Nevertheless, the degree to which these interventions can completely restore binaural auditory capabilities to improve their sound localisation ability remains contentious. This debate is primarily due to concerns regarding auditory asymmetry and signal timing discrepancies associated with these devices 31. Research by Vogt et al. 2, involving 23 participants with congenital UCHL, demonstrated that both BCD and VSB interventions could improve sound localisation accuracy as assessed through audiological evaluations. Conversely, Zhao et al. 32 documented a decrease in sound localisation accuracy after VSB fitting in individuals with congenital UCHL. In this study, when specifically examined the SSQ questionnaire, statistical analysis showed a significant improvement in the subscales of ‘speech discrimination’ and ‘spatial hearing’. The observed advancements in ‘speech discrimination’ and ‘spatial hearing’ are in line with prior investigations demonstrating notable improvement in audiological tests of speech recognition in noise and sound localisation ability 2,26. These findings suggest that patients with UCHL may exhibit more obvious improvement in sound localisation ability than what is observed in audiological tests following the use of these devices.
The APHAB questionnaire revealed a considerable decrease in mean problem percentage in the BN, EC, and RV subscales with the implementation of hearing implants (both BB and VSB groups, Figure 5). House et al. 33 studied patients with unilateral hearing loss (including UCHL, mixed hearing loss, and SNHL) who used BAHA and found less hearing problem rate in APHAB subscales with BAHA use: 17.4% in BN, 11.6% in EC, and 13.2% in RV. Limited research on using APHAB to measure hearing outcomes in UCHL patients after BB or VSB implantation hinders comparison with other studies. However, there was no improvement in the ‘quality of hearing’ subscale according to the SSQ-12 (p = 0.24, Figure 3B); moreover, the AV subscale in the APHAB exhibited a relatively higher percentage of hearing problems regarding the after implantation of BB (p < 0.05, Figure 5A) or VSB (p < 0.01, Figure 5B). Similar results were also found by House et al. 33 wherein patients with unilateral hearing loss did not show a problem decrease with the BAHA turned on for the AV subscale. The two subscales of ‘quality of hearing’ in SSQ and AV in APHAB mainly investigated negative reactions to environmental sounds. The lack of satisfaction with these outcomes can be attributed to challenges in adapting to binaural hearing after the amplification of sound from the impaired side. It is worth noting that three of the four patients who did not wear the device reported amplified sound or noise from the affected side, which led to their decision to become non-users. Unfortunately, they were all unable to come to the hospital for device fitting due to time conflicts. This highlights the importance of follow-up device fitting, particularly in addressing device-related sound and noise dissatisfaction, minimise non-usage rates, and increase subjective satisfaction.
Choice of the BB or VSB
Several aspects need to be assessed carefully when choosing the more suitable hearing implant for a patient before surgery. Firstly, the specific anatomical feature: the VSB is more suitable for those with a well-developed middle ear, particularly those with an intact stapes, while the BB has a broader range of indications 34. Furthermore, the implantation of the VSB requires a substantial degree of technical precision and expertise from the surgeon, as well as an extended duration of surgery, owing to its intricate involvement with the middle ear structures. In contrast, BB surgeries, which do not necessitate direct intervention in the middle ear, are generally less complex and time-consuming. Secondly, concerning sound quality, the literature indicates that patients with VSB implants may experience better sound signal quality compared to those with BB implants 35,36. This improvement is hypothesised to stem from the operational principles of VSB, which more closely mimic natural sound transmission pathways by specifically stimulating the impaired ear. Considering these factors, it is imperative for surgeons to thoroughly assess each patient’s unique profile and provide detailed preoperative information about the pros and cons of both implant types.
Limitations
Although the current study offers valuable insights into the effectiveness, safety, long-term usage, and subjective satisfaction of BB and VSB in patients with congenital UCHL, no significant factors (such as age, time since implantation, or daily usage time) were identified as being associated with the results of audiological outcomes and SSQ and APHAB questionnaires. This may be attributed to the limited sample size, which is a limitation that needs to be acknowledged. Subsequent research with larger sample sizes will be conducted to identify factors that impact objective and subjective outcomes of hearing implants. This can facilitate the development of more individualised implantation strategies for patients with congenital UCHL to achieve optimal hearing improvement and reduce the rate of device non-usage.
Conclusions
BB and VSB are both effective in improving the hearing of patients with congenital UCHL, with no significant difference in hearing gain between the two devices. Patients who received these hearing implants reported high levels of subjective satisfaction and low complication rates during the long-term postoperative period. However, most patients reported increased noise or sound from the atretic ear, emphasising the importance of follow-up device fitting after surgery to decrease the rate of non-usage and achieve higher levels of subjective satisfaction.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This work was supported by Capital Health Research and Development of Special (Grant No.2020-2-2057).
Author contributions
YJL, SQZ: conceived and designed the research; LY, WY: retrieved patients’ data; JKZ, WXQ, MSL: prepared figures and tables; YJL, DNW: drafted, edited, and revised the manuscript. All Authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical consideration
This study was approved by the Institutional Ethics Committee of Beijing Tongren Hospital, Capital Medical University (TRECKY2018-067). The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki. Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: June 3, 2023
Accepted: May 17, 2024
Figures and tables
Figure 1. (A) Preoperative and postoperative mean sound field hearing threshold (SFHT) at frequencies ranging from 0.25 to 4 kHz for patients with congenital UCHL and implanted with a BB or VSB; (B) Preoperative and postoperative word recognition scores (WRS) for patients with congenital UCHL implanted with BB or VSB; (C) Preoperative and postoperative speech reception threshold (SRT) for patients with congenital UCHL implanted with BB or VSB. Significant differences between pre- and postoperative scores are denoted as *p < 0.05 and **p < 0.01.
Figure 2. Individual delta scores of the Speech, Spatial and Qualities of Hearing questionnaire -12 (SSQ-12) for each patient in the (A) BB and (B) VSB groups. The SSQ-12 assesses four subscales of hearing function: speech hearing, spatial hearing, qualities of hearing, and overall score. The delta score is calculated as the difference between the score in aided condition and that in unaided condition.
Figure 3. The mean Speech, Spatial and Qualities of Hearing questionnaire-12 (SSQ-12) scores in the (A) BB and (B) VSB groups before and after device implantation. The SSQ-12 assesses four subscales of hearing function: speech hearing, spatial hearing, qualities of hearing, and overall score. Significant differences between pre- and postoperative scores are denoted as *p < 0.05 and **p < 0.01.
Figure 4. Individual delta problem percentage of the Abbreviated Profile of Hearing Aid Benefit (APHAB) for each patient in the (A) BB and (B) VSB groups. The scores are shown for four subscales: aversiveness (AV), background noise (BN), ease of communication (EC), and reverberation (RV). The delta problem percentage is calculated as the difference between the problem percentage in aided condition and that in unaided condition.
Figure 5. The mean Abbreviated Profile of Hearing Aid Benefit (APHAB) percentage in the (A) BB and (B) VSB groups before and after the hearing implantation. The results are shown for four subscales: aversiveness (AV), background noise (BN), ease of communication (EC), and reverberation (RV). Significant differences between pre- and postoperative scores are denoted as *p < 0.05 and **p < 0.01.
| Number | Sex | Side | Aetiology | Age at implantation (years) | Follow-up duration (months) | Usage time (hours/day) | Device | AC PTA of NH ear (dB HL) | BC PTA of NH ear (dB HL) | AC PTA of impaired ear (dB HL) | BC PTA of impaired ear (dB HL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | L | Atresia | 11 | 81 | 12 | BB | 15 | 15 | 61 | 9 |
| 2 | M | L | Atresia | 30 | - | 0 | BB | 8 | 8 | 82 | 20 |
| 3 | F | R | Atresia | 6 | 29 | 8 | BB | 6 | 6 | 74 | 11 |
| 4 | M | R | Atresia | 7 | 22 | 8 | BB | 6 | 6 | 74 | 11 |
| 5 | M | L | Stenosis | 11 | - | 0 | BB | 8 | 8 | 84 | 11 |
| 6 | M | R | Atresia | 6 | 25 | 8 | BB | 19 | 19 | 76 | 15 |
| 7 | F | R | Stenosis | 10 | 58 | 2 | BB | 14 | 14 | 63 | 3 |
| 8 | F | L | Atresia | 10 | 14 | 4 | BB | 17 | 17 | 79 | 17 |
| 9 | M | L | Atresia | 5 | - | 0 | BB | 13 | 13 | 71 | 15 |
| 10 | F | R | Stenosis | 7 | 14 | 6 | BB | 12 | 12 | 73 | 16 |
| 11 | M | R | Atresia | 6 | 49 | 10 | VSB | 12 | 12 | 67 | 4 |
| 12 | F | R | Atresia | 11 | 46 | 8 | VSB | 13 | 13 | 65 | 15 |
| 13 | M | R | Atresia | 35 | 41 | 10 | VSB | 21 | 21 | 97 | 31 |
| 14 | F | R | Atresia | 10 | 53 | 14 | VSB | 26 | 26 | 69 | 11 |
| 15 | M | R | Atresia | 7 | 39 | 9 | VSB | 12 | 12 | 63 | 4 |
| 16 | M | L | Atresia | 8 | 37 | 6 | VSB | 6 | 6 | 74 | 11 |
| 17 | M | R | Atresia | 7 | 37 | 6 | VSB | 12 | 12 | 63 | 7 |
| 18 | F | L | Atresia | 12 | - | 0 | VSB | 7 | 7 | 64 | 6 |
| 19 | M | L | Atresia | 13 | 29 | 4 | VSB | 9 | 9 | 73 | 9 |
| 20 | M | R | Atresia | 18 | 14 | 8 | VSB | 12 | 12 | 70 | 5 |
References
- Kesser B, Krook K, Gray L. Impact of unilateral conductive hearing loss due to aural atresia on academic performance in children. Laryngoscope. 2013;123:2270-2275. doi:https://doi.org/10.1002/lary.24055
- Vogt K, Frenzel H, Ausili S. Improved directional hearing of children with congenital unilateral conductive hearing loss implanted with an active bone-conduction implant or an active middle ear implant. Hear Res. 2018;370:238-247. doi:https://doi.org/10.1016/j.heares.2018.08.006
- Choi J, Ma S, Park H. A comparison between wireless CROS/BiCROS and soft-band BAHA for patients with unilateral hearing loss. PLoS One. 2019;14. doi:https://doi.org/10.1371/journal.pone.0212503
- Hartzell L, Chinnadurai S. Microtia and related facial anomalies. Clin Perinatol. 2018;45:679-697. doi:https://doi.org/10.1016/j.clp.2018.07.007
- Yeon E, Kim M, Im S. Chorda tympani nerve course and feasibility of its preservation during atresiaplasty for congenital aural atresia. Laryngoscope Investig Otolaryngol. 2022;7:2029-2034. doi:https://doi.org/10.1002/lio2.938
- Moon I, Byun H, Jin S. Sound localization performance improves after canaloplasty in unilateral congenital aural atresia patients. Otol Neurotol. 2014;35:639-644. doi:https://doi.org/10.1097/mao.000000000000271
- de Alarcon A, Choo D. Controversies in aural atresia repair. Curr Opin Otolaryngol Head Neck Surg. 2007;15:310-314. doi:https://doi.org/10.1097/MOO.0b013e3282f005d2
- Memari F, Mirsalehi M, Jalali A. Congenital aural atresia surgery: transmastoid approach, complications and outcomes. Eur Arch Otorhinolaryngol. 2012;269:1437-1444. doi:https://doi.org/10.1007/s00405-011-1785-6
- Hirth D, Weiss R, Stöver T. Audiological benefit and subjective satisfaction with the ADHEAR hearing system in children with unilateral conductive hearing loss. Eur Arch Otorhinolaryngol. 2021;278:2781-2788. doi:https://doi.org/10.1007/s00405-020-06364-2
- Osborne M, Child-Hymas A, Gill J. First pediatric experience with a novel, adhesive adapter retained, bone conduction hearing aid system. Otol Neurotol. 2019;40:1199-1207. doi:https://doi.org/10.1097/mao.0000000000002363
- Cadre B, Simon F, Célérier C. Long-term outcomes of retrospective case series of middle ear implantation with Vibrant Soundbridge in children with congenital aural atresia. Eur Arch Otorhinolaryngol. 2023;280:1629-1637. doi:https://doi.org/10.1007/s00405-022-07633-y
- Sprinzl G, Wolf-Magele A. The Bonebridge bone conduction hearing implant: indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol. 2016;41:131-143. doi:https://doi.org/10.1111/coa.12484
- Maw J. The Vibrant Soundbridge: a global overview. Otolaryngol Clin North Am. 2019;52:285-295. doi:https://doi.org/10.1016/j.otc.2018.11.007
- Wang D, Wang B, Ren R. Total auricular reconstruction concomitant with Bonebridge implantation using a retrosigmoid sinus approach. Acta Otorhinolaryngol Ital. 2022;142:470-475. doi:https://doi.org/10.1080/00016489.2022.2086999
- Wang Y, Xing W, Liu T. Simultaneous auricular reconstruction combined with bone bridge implantation-optimal surgical techniques in bilateral microtia with severe hearing impairment. Int J Pediatr Otorhinolaryngol. 2018;113:82-87. doi:https://doi.org/10.1016/j.ijporl.2018.07.004
- Wang D, Zhao S, Zhang Q. Vibrant Soundbridge combined with auricle reconstruction for bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol. 2016;86:240-245. doi:https://doi.org/10.1016/j.ijporl.2016.05.006
- Noble W, Jensen N, Naylor G. A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: the SSQ12. Int J Audiol. 2013;52:409-412. doi:https://doi.org/10.3109/14992027.2013.781278
- Cox R, Alexander G. The abbreviated profile of hearing aid benefit. Ear Hear. 1995;16:176-186. doi:https://doi.org/10.1097/00003446-199504000-00005
- Bruschini L, Canzi P, Canale A. Implantable hearing devices in clinical practice. Systematic review and consensus statements. Acta Otorhinolaryngol Ital. 2024;44:52-67. doi:https://doi.org/10.14639/0392-100X-N2651
- Takahashi M, Iwasaki S, Furutate S. Active middle ear implant (vibrant soundbridge) in children with unilateral congenital aural atresia. Acta Otolaryngol. 2021;141:34-38. doi:https://doi.org/10.1080/00016489.2020.1823471
- Volgger V, Schießler I, Müller J. Audiological results and subjective benefit of an active transcutaneous bone-conduction device in patients with congenital aural atresia. Eur Arch Otorhinolaryngol. 2022;279:2345-2352. doi:https://doi.org/10.1007/s00405-021-06938-8
- Stenfelt S. Transcranial attenuation of bone-conducted sound when stimulation is at the mastoid and at the bone conduction hearing aid position. Otol Neurotol. 2012;33:105-114. doi:https://doi.org/10.1097/MAO.0b013e31823e28ab
- Agterberg M, Hol M, Cremers C. Conductive hearing loss and bone conduction devices: restored binaural hearing?. Adv Otorhinolaryngol. 2011;71:84-91. doi:https://doi.org/10.1159/000323587
- Vogt K, Wasmann J, Van Opstal A. Contribution of spectral pinna cues for sound localization in children with congenital unilateral conductive hearing loss after hearing rehabilitation. Hear Res. 2020;385. doi:https://doi.org/10.1016/j.heares.2019.107847
- Van Wanrooij M, Van Opstal A. Contribution of head shadow and pinna cues to chronic monaural sound localization. J Neurosci. 2004;24:4163-4171. doi:https://doi.org/10.1523/jneurosci.0048-04-2004
- Leinung M, Zaretsky E, Lange B. Vibrant Soundbridge® in preschool children with unilateral aural atresia: acceptance and benefit. Eur Arch Otorhinolaryngol. 2017;274:159-165. doi:https://doi.org/10.1007/s00405-016-4265-1
- Crowder H, Bestourous D, Reilly B. Adverse events associated with Bonebridge and Osia bone conduction implant devices. Am J Otolaryngol. 2021;42. doi:https://doi.org/10.1016/j.amjoto.2021.102968
- Jones S, Spielmann P. Device profile of the Bonebridge bone conduction implant system in hearing loss: an overview of its safety and efficacy. Expert Rev Med Devices. 2020;17:983-992. doi:https://doi.org/10.1080/17434440.2020.1834845
- Fisch U, Cremers C, Lenarz T. Clinical experience with the Vibrant Soundbridge implant device. Otol Neurotol. 2001;22:962-972. doi:https://doi.org/10.1097/00129492-200111000-00042
- Bruchhage K, Leichtle A, Schönweiler R. Systematic review to evaluate the safety, efficacy and economical outcomes of the Vibrant Soundbridge for the treatment of sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2017;274:1797-1806. doi:https://doi.org/10.1007/s00405-016-4361-2
- Portfors C, von Gersdorff H. Macrocircuits for sound localization use leaky coincidence detectors and specialized synapses. Neuron. 2013;78:755-757. doi:https://doi.org/10.1016/j.neuron.2013.05.034
- Zhao C, Liu Y, Yang J. Sound-localisation performance in patients with congenital unilateral microtia and atresia fitted with an active middle ear implant. Eur Arch Otorhinolaryngol. 2021;278:31-39. doi:https://doi.org/10.1007/s00405-020-06049-w
- House J, Kutz J, Chung J. Bone-anchored hearing aid subjective benefit for unilateral deafness. Laryngoscope. 2010;120:601-607. doi:https://doi.org/10.1002/lary.20802
- Rahne T, Plontke S. Systematic and audiological indication criteria for bone conduction devices and active middle ear implants. Hear Res. 2022;421. doi:https://doi.org/10.1016/j.heares.2021.108424
- Liu Y, Ren R, Zhao S. Successive ipsilateral surgery of Vibrant Soundbridge and Bonebridge devices for congenital bilateral conductive hearing loss: a case report. J Int Med Res. 2020;48. doi:https://doi.org/10.1177/0300060520972280
- Monini S, Bianchi A, Talamonti R. Patient satisfaction after auditory implant surgery: ten-year experience from a single implanting unit center. Acta Otorhinolaryngol Ital. 2017;137:389-397. doi:https://doi.org/10.1080/00016489.2016.1258733
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2024 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 380 times
- PDF downloaded - 137 times



 PDF
PDF
