Head and neck
Vol. 44: Issue 4 - August 2024
Management and prognosis of iatrogenic perforations of the cervical oesophagus and hypopharynx
Abstract
Objective. The study aimed to identify factors affecting the management and prognosis of iatrogenic cervical oesophageal and hypopharyngeal perforations (iCEHPs).
Methods. We retrospectively analysed 24 patients treated for iCEHP between 2004 and 2021 at a tertiary university medical centre. Data on demographics, clinical features, imaging, management and outcomes were collected. Factors associated with primary management and patient outcome were assessed.
Results. The most common management approach was surgical neck exploration (15 patients, 62.5%). Surgical management was used in 93% of uncontained perforations compared to 11% of contained perforations (p < 0.001). Surgically-treated patients had higher levels of C-reactive protein (CRP) than conservatively-treated patients (median, 18.3 vs 4.8 mg/dL; p = 0.001). Delayed diagnosis (≥ 24 hours) was associated with increased mortality (100 vs 5%; p = 0.011). The mortality rate was significantly higher in patients who had a history of neck irradiation than in patients who did not (67 vs 5%; p = 0.032).
Conclusions. Early diagnosis of iCEHP improves outcomes. The appropriate management should be carefully selected on the basis of CRP level and imaging findings. Prior neck radiation is a poor prognostic factor.
Introduction
Cervical oesophageal and hypopharyngeal perforations (CEHPs) are relatively uncommon and therefore often under-reported 1,2. Although CEHPs have a better prognosis than distal perforations, morbidity and mortality rates can reach up to 16% 3. Extension of local and systemic infection accounts for most of the mortality risk 4. Unlike distal oesophageal perforations, the management of CEHPs remains controversial. The traditional treatment of choice is immediate cervical exploration with primary closure of the defect and transcutaneous drainage. However, recent studies support a more conservative approach in selected patients, mainly depending on perforation size and signs of systemic infection 5,6.
Iatrogenic injury is considered to be the most common cause of CEHP (58%), followed by foreign body ingestion (27%) and traumatic injuries (15%) 7. Iatrogenic injury may occur during diagnostic and therapeutic endoscopy, head and neck surgery, and cervical spine surgery, but the literature is sparse and the numbers for specific aetiologies are low 8–10. The published incidence rate of iatrogenic CEHP (iCEHP) during diagnostic and therapeutic endoscopy is less than 1%, although it is expected to rise with the growing use of these procedures 8. There is no explicit algorithm for the management of iCEHPs.
The aim of the present study was to review the factors affecting the management and prognosis of iCEHPs. The pertinent literature is reviewed.
Materials and methods
Study design and subjects
A retrospective cohort study was conducted in a tertiary university medical centre. The healthcare database was searched for all patients treated for iCEHP between 2004 and 2021, using the following ICD9 codes: 5304, 86222, 86232, 8744, 8745, 9351, Z422, and Z4223. The initial search was conducted by a single researcher with subsequent validation by a senior researcher to ensure that all identified cases conformed to the diagnostic and inclusion and exclusion criteria established for the study.
The diagnosis of iCEHP was based on findings of contrast leak, neck emphysema, and air or fluid collection on computed tomography (CT) scan or oesophagram. Inclusion criteria were age more than 18 years, confirmed radiological diagnosis of CEHP, and iatrogenic injury to the cervical oesophagus or hypopharynx. Patients with traumatic non-iatrogenic perforation, perforation of the thoracic or abdominal oesophagus, or insufficient follow-up data were excluded from the study.
Types of injury
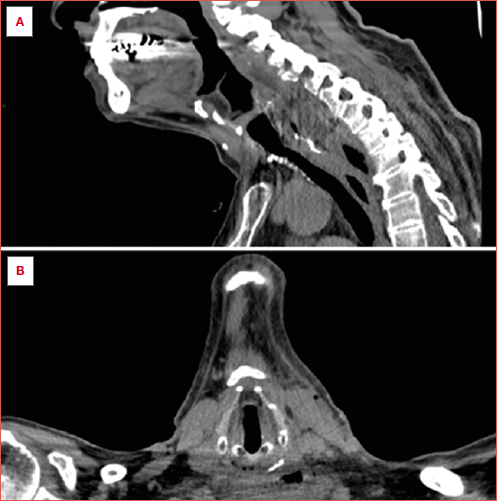
Perforations were categorised as uncontained or contained. Uncontained perforations were defined as a free extravasation of contrast agent outside the oesophageal wall or contamination of adjacent structures, including the mediastinum or neck (Cover figure). Contained perforations were defined as limited contrast leakage at the perforation site or pneumomediastinum without apparent extravasation of the contrast material 11,12.
Mechanisms of injury
Mechanisms of injury were categorised as intraluminal and extraluminal. Intraluminal mechanisms of injury included procedures performed within the oesophagus: rigid and flexible diagnostic endoscopy with or without biopsy, foreign body removal using rigid or flexible esophagoscopy, endoscopic Zenker’s diverticulum surgery, and nasogastric tube insertion. Extraluminal mechanisms of injury included procedures originating outside the oesophagus: open Zenker’s diverticulum surgery, cervical spine surgery, and thyroidectomy. In all extraluminal procedures, a nontraumatic nasogastric tube was inserted at onset to better identify the oesophagus.
Types of management
The choice of conservative or surgical management of iCHEP was based on the clinical, laboratory, and perforation characteristics. Conservative management included nil per os, administration of intravenous fluids and antibiotics, and close follow-up. Surgical treatment consisted of neck exploration of the oesophageal compartment, necrotic tissue debridement, copious irrigation with saline and antibiotics, and placement of neck and/or chest drainages. When the perforation was not identified in the operating room, management included repeated drainage and irrigation. When the perforation was identified in the operating room, management included repeated drainage, irrigation, and primary repair with two-layer closure.
If the clinical, laboratory, or imaging findings failed to adequately improve, further surgical treatment was attempted.
Data collection
Data on demographics, clinical features, laboratory findings, type of management, and outcomes were collected from electronic medical records. Laboratory parameters included white blood cell (WBC) count (normal range 4-11 x 103/μL) and C-reactive protein (CRP) level (normal level < 0.5 mg/dL). Comorbidity was evaluated with the Charlson Comorbidity Index (CCI) which summarises the most severe conditions in the individual patient and yields a summary score 13. The primary outcome measure of the study was mortality, defined as death during hospitalisation or within 30 days of discharge. The secondary outcome measures were complications appearing after primary management of the perforation and length of hospital stay. Complications included sepsis, pharyngocutaneous fistula formation, pneumonia, mechanical ventilation or need for tracheostomy, pleural effusion, pneumothorax, and mediastinitis. Sepsis was defined as suspected infection based on an increase of ≥ 2 points on the Sequential Organ Failure Assessment (SOFA), used to evaluate respiration, coagulation, liver, cardiovascular, and central nervous system dysfunction 14.
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences (SPSS), v.23.0 (IBM Corp. Armonk, NY). Associations between categorical variables were analysed using Fisher’s exact test because the expected cell count was less than 5. Associations between continuous and categorical variables were analysed using the Mann-Whitney nonparametric test for non-normally distributed data. Histograms, Q-Q plots, and on-sample Kolmogorov-Smirnov test were used to test the normality of the continuous variables. A two-sided p-value of < 0.05 was considered significant.
Results
Demographic and clinical characteristics
The study group included 24 patients with iCEHP, 15 males and 9 females (male to female ratio, 1.7:1) with a median age of 69 years (range 29-89) at diagnosis. The clinical characteristics of the study population are summarised in Table I. Eighteen patients (75%) scored > 2 on the CCI. A history of smoking was documented in 8 patients (33%), diabetes in 8 (33%), and prior neck radiation in 3 (12.5%). Cervical pain (17 patients, 71%) and dysphagia (8 patients, 33%) were the most common presenting symptoms. Eighteen perforations (75%) were caused by intraluminal interventions and 6 (25%) by extraluminal interventions. The main causes of perforation (Fig. 1) were foreign body removal (7 patients, 29%), diagnostic endoscopy (6 patients, 25%), and repair of Zenker’s diverticulum (endoscopic or open approach) (4 patients, 17%).
Twenty-two patients (92%) underwent diagnostic CT of the neck and chest with oral contrast; 4 patients (17%) underwent oesophagography. Twenty perforations (83%) were located in the cervical oesophagus and 4 in the hypopharynx (17%). Nine perforations (37.5%) were contained and 15 (62.5%) were uncontained.
Management
Conservative management was practiced in 9 patients (37.5%), neck exploration in 9 (37.5%), and exploration with primary repair in 6 (25%). Signs and symptoms of perforation were evident within a median of 20 hours from injury (range, 1 hour-2 weeks). The median interval from clinical presentation to diagnosis of perforation was 11.5 hours (range, 3-72 hours), and from diagnosis to primary surgical management, 12 hours (range, 1-96 hours). Following diagnosis, patients were kept nil per os for a median duration of 10 days (range, 4-32 days). Intravenous antibiotics were administered for a median of 7.5 days (range, 7-14 days) in patients treated conservatively and a median of 16 days (range, 7-32 days) in patients treated surgically (p = 0.009).
Associations of patient and injury characteristics with type of primary management are presented in Table I. Uncontained perforations were significantly associated with surgical management compared to contained perforations (93% vs. 11%; p < 0.001). Surgically treated patients presented with a higher CRP level (median 18.3, range 4.3-33 mg/dL) than conservatively managed patients (median 4.8, range 1.3-5.9 mg/dL; p = 0.001). There were no significant correlations between patient age, background diseases, or laboratory parameters and type of primary management.
Ultimately, 16 patients (67%) underwent surgical intervention (Fig. 2). Surgery was performed for all perforations caused by extraluminal interventions (6/6) and 55.5% of those caused by intraluminal interventions (10/18; p = 0.066). All perforations related to spinal surgery (2/2) were managed by exploration with primary closure compared to 18% of perforations for other causes (p = 0.054). There were no other associations between patient characteristics and primary closure during surgery.
Primary outcomes
Three patients (12.5%) died from complications of septic shock. In the first patient with history of neck radiation, perforation was caused by nasogastric tube insertion. There were no clinical symptoms or leukocytosis, and the diagnosis was made 5 days after injury by review of the CT scan. The patient underwent neck exploration at 96 hours after diagnosis. The second patient was diagnosed 36 hours after open repair of Zenker diverticulum. Primary conservative treatment failed and was followed by repeated cervical and thoracic explorations. The perforation was eventually located and repaired. In the third patient, hypopharyngeal perforation occurred during endoscopic biopsies for recurrent hypopharyngeal cancer following prior radiotherapy. There was no delay in diagnosis or neck exploration. All three patients who died needed secondary management with thoracotomy with drainage due to mediastinitis. Subsequent general deterioration did not allow for further intervention.
Survival and prognostic factors are presented in Table II. Mortality was significantly related to previous radiation therapy (p = 0.032), delayed diagnosis (≥ 24 hours from presentation to diagnosis) (p = 0.011), and WBC count at presentation (median, 4.8 vs 12.3 x 103/μL; p = 0.001). Rates of previous radiation therapy were 67% in patients who died (2/3) compared to 5% in survivors (p = 0.032). Mortality rates were 100% in patients with a delayed diagnosis compared to 5% in patients diagnosed within 24 hours from presentation (p = 0.011). Patients who died were diagnosed at a median of 36 hours (range, 14-72) from presentation compared to 11 hours (range, 3-22) in survivors (p = 0.041). Those with a delayed diagnosis presented with normal WBC count relative to patients who presented with leukocytosis and diagnosed within 24 hours (median, 5.8 vs 12.5 x 103/μL; p = 0.029). Furthermore, the patients who died presented with normal WBC count (median 4.8, range 4.1-6.7 x 103/μL) and demonstrated leukocytosis only after initiation of treatment (median 13.1, range 5.1-13.6 x 103/μL). Conversely, surviving patients presented with leukocytosis (median 12.3, range 7.5-19.8 x 103/μL) that normalised after treatment initiation (median 7.3, range 4.8-13.4 x 103/μL). There was no association of the other patient characteristics with survival.
Secondary outcomes
Treatment outcomes are presented in Table III. The median duration of hospitalisation was 11.5 days (range, 4-47). Hospitalisation time was significantly longer in patients with uncontained than contained perforations (median, 19 days vs 7 days; p < 0.001) and in patients treated surgically than conservatively (median, 20 days vs 7 days; p < 0.001). It was also longer in patients with perforations related to spinal surgery than in patients with perforations of other aetiologies (median 44.5 days vs 11 days; p = 0.029).
Complications were reported in 11 patients (46%). The overall complication rate was not associated with type of primary management (surgical or conservative; 53 vs 33%; p = 0.423), other signs and symptoms, or hospitalisation time.
Discussion
We present one of the largest series of iCEHP in the literature. This study sought to investigate the prognostic factors and appropriate management of iCEHP, which have been scarcely reported to date 1,2. The findings have important clinical implications given the life-threatening nature of these injuries.
Most of patients in our cohort (63%) were treated surgically with or without primary repair of the defect. The results suggest that imaging findings and CRP levels may play a crucial role in iCEHP management decisions.
A few studies have proposed criteria for consideration when deciding on the therapeutic approach to cervical oesophageal perforations. Zenga et al. 15, in a study of 28 patients with iatrogenic and non-iatrogenic CEHPs, half of whom were managed surgically, suggested three conditions for neck exploration with drainage: delayed diagnosis (≥ 24 hours), ingestion of food before diagnosis, and signs of sepsis. Others focused on injuries following cervical spine surgery and proposed that conservative treatment may be used in cases of small (< 2 cm), early-diagnosed perforations in the absence of signs or symptoms of infection 16,17.
Studies of thoracic and abdominal oesophageal perforations showed that imaging findings are associated with the size and severity of the perforation and suggested that management be determined accordingly 12,18. In a recent review, Lampridis et al. 19 proposed reserving conservative treatment for contained perforations limited to the oesophageal wall. However, this classification is not supported in CEHPs. The present study highlights the important role of imaging in assessing injury severity in all iCEHPs and specifically to determine whether perforations are contained or uncontained. While uncontained perforations should be treated with neck exploration with or without primary closure, conservative management may suffice for contained perforations in patients with no evidence of systemic inflammation.
CRP is an acute-phase reactant that increases within 2 hours and peaks at 48 hours in response to trauma and inflammatory conditions 20. Lausevic et al. 21 evaluated 75 patients with trauma and found that those who had multiple organ failure had higher CRP levels on the first day than those who did not. They concluded that CRP may serve as a good early marker in this setting. These results were supported by another study of 313 intensive care unit patients that suggested that CRP levels may be helpful to identify those who require more aggressive management 22. Accordingly, we found that CRP level at presentation was related to the therapeutic approach selected: patients with high CRP levels were treated surgically whereas those with low CRP levels were managed conservatively. Thus, high CRP levels at presentation may indicate the need for surgical treatment. In our cohort, all perforations caused by extraluminal interventions were managed surgically, in contrast to 55.5% of perforations caused by intraluminal interventions. However, the correlation between the aetiology of the perforation and its management was only marginally significant. Fetterman et al. 23 reviewed the data on 48 patients with traumatic hypopharyngeal injury and concluded that the mechanism of injury is as important to the management decision as the size and location of the perforation. Other investigations of injuries caused by cervical spine surgery found that forceful retraction, pressure applied on the oesophageal wall, and the use of sharp metal devices increased the risk of severe perforation during extraluminal interventions 17. Additional studies are required to determine the manner in which the aetiology of the perforation affects its severity and, consequently, the choice of management.
Three of the 24 patients (12.5%) died of complications of iCEHP. There was no association of treatment modality with complication and survival rates. However, surgical treatment and uncontained perforation correlated with a significantly longer duration of hospitalisation. This finding was probably a consequence of the severity of the disease at presentation rather than the choice of treatment modality for the perforation. However, Zenga et al. 15 found no significant difference in median hospitalisation time between conservatively-managed and surgically-treated patients (13 days vs 12.5 days), with a higher complication rate in patients treated by exploration with primary repair.
Previous studies suggested several risk factors for iCEHP, including head and neck malignancy, kyphosis, anatomical abnormalities, and anterior cervical pathologies 1,12. The present study found that prior neck radiation was a significant risk factor for mortality in patients with iCEHP.
In addition, delayed diagnosis contributed to poor outcome. Two of the three patients who died were diagnosed ≥ 24 hours after injury. The data in the literature on the relationship between delayed diagnosis of oesophageal perforation and prognosis are inconclusive. While most studies that examined iatrogenic or non-iatrogenic CEHPs suggested that prognosis is related to the time between injury and diagnosis 4,16,17, others found that the time from injury to diagnosis may not be as important as commonly believed 24.
We noted that WBC count at presentation was not a reliable indicator of either the presence or the severity of iCEHPs, and that normal WBC count at presentation may lead to a delay in diagnosis, resulting in poor outcome. Previous studies of all-body traumatic injury reached similar conclusions 25. Chang et al. 26 investigated 882 trauma patients and reported no difference in WBC count at admission between those who were surgically or conservatively treated. They concluded that it may be the variation in WBC count during hospitalisation rather than the WBC count at admission that is clinically important. Similarly, the results of our study indicate that physicians should maintain a high index of suspicion and not rely solely on the WBC count at presentation to confirm or refute serious injury, especially in patients with a history of neck radiation.
One of the primary limitations of our study is the small sample size, which is attributable mainly to the rarity of iCEHP. Although we described one of the largest series of patients with iCEHP to date, which was sufficient for statistical analysis, logistic regression analysis was not applicable. Therefore, the results rely solely on association analysis, underscoring the necessity for further research to corroborate the observed trends and patterns.
The study is also limited by its retrospective design. Relying on existing data, which can be incomplete or vary in quality, is a challenge that is commonly encountered in retrospective studies. Nevertheless, we believe our careful review of the medical records and the large amount of data available are adequate to provide valuable information for clinicians who manage iCEHPs. Larger prospective studies are needed to determine the conditions under which clinicians should consider converting from conservative to surgical management.
Conclusions
iCEHPs are rare and pose a diagnostic and therapeutic challenge. Early diagnosis is essential for the success of treatment. WBC count at presentation is not a reliable indicator of the presence or severity of injury. Prior neck radiation is a significant risk factor for poor prognosis. Our study suggests that high CRP levels at presentation and uncontained perforation on imaging indicate the need for primary surgical management.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
EGL, AM, YH: conceptualisation; EGL, AR, AM, YH: methodology; EGL, AR: formal analysis; EGL, AR, AM, YH: validation; EGL, AR: data curation; EGL: writing-original draft preparation; EGL, AR, AM, AA, TS, GB, YH: writing-review and editing; EGL, YH: visualisation; YH: supervision; EGL, YH: project administration. All authors have read and agreed to the published version of the manuscript.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Rabin Medical Center (0414-16-RMC, July 2018).
Patient consent was waived due to the retrospective nature.
History
Received: January 12, 2024
Accepted: March 8, 2024
Figures and tables
Figure 1. Distribution of causes of iatrogenic injuries to the cervical oesophagus and hypopharynx.
Figure 2. Final management of iatrogenic perforations of the cervical oesophagus and hypopharynx by mechanism of injury. A) Perforations due to intraluminal interventions; (B) Perforations due to extraluminal interventions. ZD: Zenker’s diverticulum. Note that one patient with oe- sophageal perforation due to open Zenker’s diverticulum surgery was initially managed conservatively, but eventually required neck exploration because of worsening clinical and laboratory parameters.
| Characteristics | All patients | Conservative management | Surgical management | P |
|---|---|---|---|---|
| (n = 24) | (n = 9) | (n = 15) | ||
| Male sex, n (%) | 15 (62.5) | 5(56) | 10(67) | 0.678 |
| Age, median (range), years | 69 (29-89) | 74 (54-89) | 68 (29-79) | 0.347 |
| Comorbidity, n (%) | ||||
| Charlson Comorbidity Index > 2 | 18(75) | 7(78) | 11(73) | > 0.99 |
| Smoking history | 8(33) | 4(44) | 4(27) | 0.412 |
| Diabetes | 8(33) | 2(22) | 6(40) | 0.657 |
| Neck comorbidity, n (%) | ||||
| Neck radiation | 3 (12.5) | 1(11) | 2(13) | > 0.99 |
| Cervical malignancy | 2(8) | 0 | 2(12) | 0.511 |
| Prior Zenker’s diverticulum surgery | 2(8) | 1(11) | 1(7) | > 0.99 |
| Prior cervical spine surgery | 2(8) | 0 | 2(13) | 0.511 |
| Prior thyroidectomy | 1(4) | 0 | 1(7) | > 0.99 |
| Presenting signs and symptomsa | ||||
| Cervicalgia, n (%) | 17(71) | 6(67) | 11(73) | > 0.99 |
| Dysphagia, n (%) | 8(33) | 5(56) | 3(20) | 0.99 |
| Neck crepitations, n (%) | 5(21) | 2(22) | 3(20) | > 0.99 |
| Neck swelling, n (%) | 3 (12.5) | 1(11) | 2(13) | > 0.99 |
| WBC count (103/μL), median (range) | 11.7 (4.1-19.8) | 10.8 (6.7-19.8) | 12.4 (4.1-16.9) | 0.446 |
| CRP (mg/dL), median (range) | 11.3 (1.3-33) | 4.8 (1.3-5.9) | 18.3 (4.3-33) | 0.001* |
| Fever >38°C, n (%) | 6(25) | 1(11) | 5(33) | 0.351 |
| Heart rate >100 bpm, n (%) | 4(17) | 1(11) | 3(20) | > 0.99 |
| Imaging findings at diagnosis, n (%) | < 0.001b* | |||
| Contained perforation | 9 (37.5) | 8(89) | 1(7) | |
| SCE with limited contrast leakage to perforation site | 4 (16.5) | 3(33) | 1(7) | |
| SCE and/or PM without apparent extravasation | 5(21) | 5(56) | 0 | |
| Uncontained perforation | 15 (62.5) | 1(11) | 14(93) | |
| Extravasation outside the oesophagus | 8(33) | 1(11) | 7 (46.5) | |
| Mediastinitis with SCE and PM | 4(17) | 0 | 4 (26.5) | |
| Retropharyngeal fluid collection with gas bubbles inside and SCE | 3 (12.5) | 0 | 3(20) | |
| Mechanism of injuryc, n (%) | 0.351 | |||
| Intraluminal intervention | 18(75) | 8(89) | 10(67) | |
| Extraluminal intervention | 6(25) | 1(11)d | 5(33) | |
| Location of perforation, n (%) | 0.259 | |||
| Cervical oesophagus | 20(83) | 9(100) | 11(73) | |
| Hypopharynx | 4(17) | 0 | 4(100) | |
| WBC: white blood cell; CRP: C-reactive protein; SCE: subcutaneous emphysema; PM: pneumomediastinum. | ||||
| *Statistically significant. | ||||
| a Presenting signs and symptoms were identified within 24 hours after perforation. | ||||
| b Comparison between contained and uncontained perforations. | ||||
| c Intraluminal aetiologies included endoscopic Zenker’s diverticulum surgery, foreign body removal, diagnostic endoscopy, endoscopic biopsy, and nasogastric tube insertion. | ||||
| Extraluminal aetiologies included open Zenker’s diverticulum surgery, cervical spine surgery, and thyroidectomy. | ||||
| d One patient with oesophageal perforation caused by open Zenker’s diverticulum surgery was initially managed conservatively but eventually required neck exploration because of worsening clinical and laboratory parameters. | ||||
| Characteristics | Survived | Died | P |
|---|---|---|---|
| (n = 21) | (n = 3) | ||
| Male sex, n (%) | 14(67) | 1(33) | 0.533 |
| Age (years), median (range) | 69 (29-89) | 68 (59-77) | > .99 |
| Comorbidity, n (%) | |||
| Charlson Comorbidity Index >2 | 15(71) | 3(100) | 0.546 |
| Smoking history | 7(33) | 1(33) | > 0.99 |
| Diabetes | 6(29) | 2(67) | 0.249 |
| Neck comorbidity, n (%) | |||
| Neck radiation | 1(5) | 2(67) | 0.032* |
| Cervical malignancy | 1(5) | 1(33) | 0.239 |
| Prior Zenker’s diverticulum surgery | 1(5) | 1(33) | 0.239 |
| Prior cervical spine surgery | 2 (9.5) | 0 | > 0.99 |
| Prior thyroidectomy | 1(5) | 0 | > 0.99 |
| Presenting signs and symptomsa | |||
| Cervicalgia, no. (%) | 16(76) | 1(33) | 0.55 |
| Dysphagia, no. (%) | 8(38) | 0 | 0.526 |
| Neck crepitations, no. (%) | 4(19) | 1(33) | 0.521 |
| Neck swelling, no. (%) | 3(14) | 0 | > 0.99 |
| WBC count (103/μL), median (range) | 12.3 (7.5-19.8) | 4.8 (4.1-6.7) | 0.001* |
| CRP (mg/dL), median (range) | 6.1 (1.3-33) | 11 (4.8-31) | 0.505 |
| Fever > 38°C, n (%) | 4(19) | 2(67) | 0.143 |
| Heart rate > 100 bpm, n (%) | 3(14) | 1(33) | 0.437 |
| Imaging findingsb, n (%) | > 0.99 | ||
| Contained perforation | 8(38) | 1(33) | |
| Uncontained perforation | 13(62) | 2(67) | |
| Mechanism of injuryc | 0.579 | ||
| Intraluminal intervention | 15(71) | 2(67) | |
| Extraluminal intervention | 6(29) | 1(33) | |
| Location of perforation, n (%) | 0.437 | ||
| Cervical oesophagus | 18(86) | 2(67) | |
| Hypopharynx | 3(14) | 1(33) | |
| Time from injury to diagnosis, n (%) | 0.011* | ||
| < 24 hours | 21(100) | 1(33) | |
| ≥ 24 hours | 0 | 2(67) | |
| Hospital stay (days), median (range) | 11 (4-46) | 32 (10-47) | 0.202 |
| Complications, n (%) | 8(38) | 3(100) | 0.082 |
| WBC: white blood cell; CRP: C-reactive protein. | |||
| *Statistically significant. | |||
| a Presenting signs and symptoms were identified within 24 hours after perforation. | |||
| b A contained perforation was defined as limited contrast leakage at the perforation site or pneumomediastinum without apparent extravasation of the contrast material. An uncontained perforation was defined as a free extravasation of contrast agent outside the oesophageal wall or contamination of adjacent structures, including mediastinum or neck. | |||
| c Intraluminal aetiologies included endoscopic Zenker’s diverticulum surgery, foreign body removal, diagnostic endoscopy, endoscopic biopsy and nasogastric tube insertion. Extraluminal aetiologies included open Zenker’s diverticulum surgery, cervical spine surgery and thyroidectomy. | |||
| Characteristics | All patients | Conservative management | Surgical management | P |
|---|---|---|---|---|
| (n = 24) | (n = 9) | (n = 15) | ||
| Mortality, n (%) | 3 (12.5) | 1(11) | 2(13) | > 0.99 |
| Time from injury to diagnosis, n (%) | > 0.99 | |||
| < 24 hours | 22(92) | 8(89) | 14(93) | |
| ≥ 24 hours | 2(8) | 1(11) | 1(7) | |
| Feeding following perforation, n (%) | > 0.99 | |||
| No feeding | 7(29) | 5(56) | 2(13) | |
| Nasogastric tube | 11(46) | 2(22) | 9(60) | |
| Parenteral nutrition | 3 (12.5) | 1(11) | 2(13) | |
| Gastrostomy tube | 3 (12.5) | 1(11) | 2(13) | |
| Hospital stay (days), median (range) | 11.5 (4-47) | 7 (4-11) | 20 (9-47) | < 0.001* |
| Complicationsa, n (%) | 11(46) | 3(33) | 8(53) | 0.423 |
| Pneumonia | 5(21) | 0 | 5(33) | |
| Pharyngocutaneous fistula | 4(17) | 1(11) | 3(20) | |
| Sepsis | 4(17) | 1(11) | 3(2) | |
| Mediastinitis | 3 (12.5) | 1(11) | 2(13) | |
| Mechanical ventilation | 3 (12.5) | 1(11) | 2(13) | |
| Tracheostomy | 3 (12.5) | 1(11) | 2(13) | |
| Pneumothorax | 2(8) | 0 | 2(13) | |
| Pleural effusion | 2(8) | 1(11) | 1(7) | |
| *Statistically significant. | ||||
| a Complications that appeared after primary management. | ||||
References
- Chen S, Shapira-Galitz Y, Garber D. Management of iatrogenic cervical esophageal perforations: a narrative review. JAMA Otolaryngol Head Neck Surg. 2020;146:488-494. doi:https://doi.org/10.1001/jamaoto.2020.0088
- Goudy S, Miller F, Bumpous J. Neck crepitance: evaluation and management of suspected upper aerodigestive tract injury. Laryngoscope. 2002;112:791-795. doi:https://doi.org/10.1097/00005537-200205000-00005
- Kaman L, Iqbal J, Kundil B. Management of esophageal perforation in adults. Gastroenterol Res. 2010;3. doi:https://doi.org/10.4021/gr263w
- Onat S, Ulku R, Cigdem K. Factors affecting the outcome of surgically treated non-iatrogenic traumatic cervical esophageal perforation: 28 years experience at a single center. J Cardiothorac Surg. 2010;5. doi:https://doi.org/10.1186/1749-8090-5-46
- Niezgoda J, McMenamin P, Graeber G. Pharyngoesophageal perforation after blunt neck trauma. Ann Thorac Surg. 1990;50:615-617. doi:https://doi.org/10.1016/0003-4975(90)90199-G
- Dolgin S, Kumar N, Wykoff T. Conservative medical management of traumatic pharyngoesophageal perforations. Ann Otol Rhinol Laryngol. 1992;101:209-215. doi:https://doi.org/10.1177/000348949210100303
- Sdralis E, Petousis S, Rashid F. Epidemiology, diagnosis, and management of esophageal perforations: systematic review. Dis Esophagus. 2017;30:1-6. doi:https://doi.org/10.1093/dote/dox013
- Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc. 2001;53:620-627. doi:https://doi.org/10.1067/mge.2001.114422
- von Rahden B, Stein H, Scherer M. Late hypopharyngo-esophageal perforation after cervical spine surgery: proposal of a therapeutic strategy. Eur Spine J. 2005;14:880-886. doi:https://doi.org/10.1007/s00586-005-1006-3
- Peretti G, Piazza C, Del Bon F. Endoscopic treatment of Zenker’s diverticulum by carbon dioxide laser. Acta Otorhinolaryngol Ital. 2010;30:1-4.
- Abbas G, Schuchert M, Pettiford B. Contemporaneous management of esophageal perforation. Surgery. 2009;146:749-756. doi:https://doi.org/10.1016/j.surg.2009.06.058
- Brinster C, Singhal S, Lee L. Evolving options in the management of esophageal perforation. Ann Thorac Surg. 2004;77:1475-1483. doi:https://doi.org/10.1016/j.athoracsur.2003.08.037
- Austin S, Wong Y, Uzzo R. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53:E65-E72. doi:https://doi.org/10.1097/MLR.0b013e318297429c
- Singer M, Deutschman C, Seymour C. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801-810. doi:https://doi.org/10.1001/jama.2016.0287
- Zenga J, Kreisel D, Kushnir V. Management of cervical esophageal and hypopharyngeal perforations. Am J Otolaryngol. 2015;36:678-685. doi:https://doi.org/10.1016/j.amjoto.2015.06.001
- Kang M, Kim K, Park J. Management of esophageal and pharyngeal perforation as complications of anterior cervical spine surgery. World Neurosurg. 2017;102:275-283. doi:https://doi.org/10.1016/j.wneu.2017.02.130
- Harman F, Kaptanoglu E, Hasturk A. Esophageal perforation after anterior cervical surgery: a review of the literature for over half a century with a demonstrative case and a proposed novel algorithm. Eur Spine J. 2016;25:2037-2049. doi:https://doi.org/10.1007/s00586-016-4394-7
- Bufkin B, Miller J, Mansour K. Esophageal perforation: emphasis on management. Ann Thorac Surg. 1996;61:1447-1452. doi:https://doi.org/10.1016/0003-4975(96)00053-7
- Lampridis S, Mitsos S, Hayward M. The insidious presentation and challenging management of esophageal perforation following diagnostic and therapeutic interventions. J Thorac Dis. 2020;12:2724-2734. doi:https://doi.org/10.21037/jtd-19-4096
- Landry A, Docherty P, Ouellette S. Causes and outcomes of markedly elevated C-reactive protein levels. Can Fam Physician. 2017;63:E316-E323.
- Lausevic Z, Lausevic M, Trbojevic-Stankovic J. Predicting multiple organ failure in patients with severe trauma. Can J Surg. 2008;51:97-102.
- Lobo S, Lobo F, Bota D. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123:2043-2049. doi:https://doi.org/10.1378/chest.123.6.2043
- Fetterman B, Shindo M, Stanley R. Management of traumatic hypopharyngeal injuries. Laryngoscope. 1995;105:8-13. doi:https://doi.org/10.1288/00005537-199501000-00005
- Bhatia P, Fortin D, Inculet R. Current concepts in the management of esophageal perforations: a twenty-seven year Canadian experience. Ann Thorac Surg. 2011;92:209-215. doi:https://doi.org/10.1016/j.athoracsur.2011.03.131
- Paladino L, Subramanian R, Bonilla E. Leukocytosis as prognostic indicator of major injury. West J Emerg Med. 2010;11:450-455.
- Chang D, Cornwell E, Phillips J. Early leukocytosis in trauma patients: what difference does it make?. Curr Surg. 2003;60:632-635. doi:https://doi.org/10.1016/j.cursur.2003.07.011
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2024 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 233 times
- PDF downloaded - 55 times




 PDF
PDF
