Rhinology
Vol. 45: Issue 1 - February 2025
Blood and local nasal eosinophilia in chronic rhinosinusitis with nasal polyps: prevalence and correlation with severity of disease
Abstract
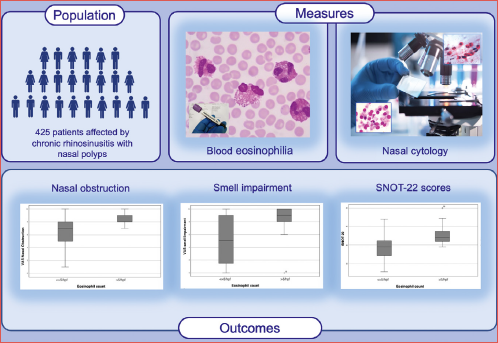
Objective. The aim of this study was to evaluate the clinical relevance of absolute eosinophil blood count and eosinophil count by nasal cytology in the context of real-life clinical practice and to determine if they correlate with the severity of symptoms in patients with chronic rhinosinusitis with nasal polyps (CRSwNP).
Methods. We enrolled 425 patients with CRSwNP followed between January 2015 and April 2023 at the A. Gemelli Hospital Foundation-IRCCS, Rome, Italy. We gathered data on blood and local eosinophil count and correlated the results with clinical features. All patients underwent endoscopy, Visual Analogical Scale (VAS) for main symptoms, and SinoNasal Outcome Test 22 (SNOT-22).
Results. We observed significantly higher mean levels of eosinophils in serum and at nasal cytology in patients with CRSwNP and comorbidities (asthma, non-steroidal anti-inflammatory drugs - exacerbated respiratory disease and allergy) compared to those without. Blood eosinophilia was not associated with severity of symptoms, whereas patients with nasal eosinophil count > 5 eosinophils per high-power field at nasal cytology had a significantly higher median specific VAS for nasal symptoms and significantly higher SNOT 22 scores.
Conclusions. We demonstrated that blood eosinophil count and nasal cytology may represent useful tools in routine clinical practice to define Type 2 inflammation and that their levels are usually higher in patients with comorbidities. We also showed that blood eosinophilia was not correlated with severity of symptoms, whereas local eosinophil count was associated with high severity of symptoms and high burden on quality of life.
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disease of sinonasal mucosa that may or may not be associated with nasal polyps (NP). In Western countries, about 80% of cases of chronic rhinosinusitis with nasal polyps (CRSwNP) are characterised by a predominantly Type 2 response, driven by proinflammatory cytokines such as IL-5, IL-4, and IL-13, alongside high levels of eosinophils in surrounding tissue. At sites of inflammation, they release inflammatory mediators that contribute to the innate immune response and disease pathogenesis, including excessive tissue remodelling. In these patients, elevated serum levels of eosinophils may also be observed, which may be predictive of a Type 2 endotype 1-4.
Many studies have demonstrated that tissue eosinophilia correlates with prognosis and severity of disease; the presence of mucosal eosinophilia, in fact, is frequently associated with more severe disease and re-appearance of nasal polyps after surgery 5. For this reason, it is important to identify early eosinophilic CRSwNP in order to understand the natural course of disease and risk of worsening, with significant therapeutic and prognostic implications, make decisions on pharmacotherapy and extent of surgery, and determine eligibility for biological therapy 3-6.
In this regard, methods used to study tissue eosinophilia play a crucial role. There is extreme heterogeneity in sampling methods that may include collecting nasal secretions, brushing, scraping of nasal mucosa, and biopsy of the inferior turbinate or the nasal polyps 7-8. Biopsies of nasal mucosa allow accurate definition of the eosinophilic infiltrate and a value ≥ 10 eosinophils counted under a high-power field (hpf, ×400) is currently considered suggestive of Type 2 inflammation 3-9. Nasal cytology has also been used in clinical practice in recent years to evaluate local eosinophilia in patients with CRS. Although local eosinophilia at nasal cytology has been extensively studied as a marker of Type 2 inflammation and as a predictor of disease severity, there is still no agreement on cut-off values 8-10.
The aim of this study was to evaluate the clinical significance of absolute eosinophil blood count and eosinophil count in nasal cytology in the context of real-life clinical practice. More specifically, we tried to determine if these may be useful to define Type 2 inflammation and if there is an association to severity of symptoms and impairment of quality of life in patients with CRSwNP.
Materials and methods
Study population, inclusion and exclusion criteria
This is an observational, cross-sectional non-profit study. We included 425 patients with CRSwNP (mean age: 51.9 years; range 23-75, female to male ratio = 0.8:1). Patients were followed between January 2015 and April 2023 at the A. Gemelli Hospital Foundation-IRCCS, Catholic University of Sacred Heart, Rhinology Unit, Rome, Italy.
Inclusion criteria were: confirmed diagnosis of diffuse CRSwNP by endoscopy and CT performed within 6 months prior to baseline evaluation; patients who performed an additional work-up in the last 3 months including evaluation by a pulmonologist and allergologist along with blood test with absolute eosinophil blood count; no use of intranasal or systemic corticosteroids within one month prior to inclusion and presence of significant symptoms of CRSwNP including nasal obstruction, rhinorrhoea, and loss of smell.
Exclusion criteria were: primary localised CRS; CRSwNP previously treated or under treatment with biologics; previous immunotherapy, acute exacerbation of CRS as defined in the EUFOREA/EPOS guidelines 1; secondary diffuse or localised CRS (cystic fibrosis, sinonasal tumours, primary ciliary dyskinesia, or autoimmune disease); allergic fungal rhinosinusitis; continuous systemic steroid treatment; sinonasal granulomatous disease and sinonasal tumour; previous radiotherapy for head and neck cancer.
Demographic characteristics were collected including age, gender, asthma, non-steroidal anti-inflammatory drugs - exacerbated respiratory disease (NSAID-ERD), smoking, number of short cycles of oral corticosteroids during previous year, and number of previous surgeries (Tab. I).
Informed consent about privacy and utilisation of clinical data was obtained from all patients at the time of baseline data collection. Clinical data were anonymously analysed.
Study design
At baseline we collected information about previous treatments including endoscopic surgeries and brief cycles of systemic steroids (cycles with > 5 and < 21 days of systemic corticosteroids in the last year) or long-term steroid use. Images from a recent CT scan were analysed to confirm the diagnosis of diffuse CRS.
Furthermore, we gathered information on the following clinical data:
- Evaluation of absolute eosinophil blood count expressed as cells/μL. A cut-off of 150 cell/mm3, as defined by last EUFORA/EPOS update1, was chosen to define Type 2 inflammation-related disease;
- Nasal endoscopy to confirm the presence of nasal polyps at baseline;
- Information on asthma (based on evaluation by pulmonologist and respiratory functional evaluation);
- Allergy information based on allergological assessment. All patients underwent allergometric skin tests for at least 18 common inhalant allergens, total immunoglobulin E (IgE) PRIST and serum specific IgE levels;
- NSAID-ERD (based on reported history of adverse reactions associated with aspirin and/or other non-steroidal anti-inflammatory drugs or on allergology evaluation);
- Specific symptoms (rhinorrhoea, nasal obstruction, smell impairment, watery eyes) were analysed by Visual Analogical Scale (VAS). For quality of life, we used the validated Italian version of SinoNasal Outcome Test 22 (SNOT-22), with a possible total score range of 0-110 11,12;
- Evaluation of local eosinophilia by nasal cytology: nasal leukocyte count was performed on scraped nasal tissue obtained from the inferior turbinate bilaterally. Scraping was performed with a rhinoprobe (Farmark s.n.c, Milan, Italy) according to previous experience 7-9. The sample was gently spread on glass slides and immediately fixed in 95% ethyl alcohol and stained with May-Grunwald-Giemsa. The slides were examined under oil immersion by light microscopy first at a magnification of 400× and then at a magnification of 1000×. Nasal tissue eosinophil infiltration was measured as eosinophil count per high power field (Ec-hpf) and reported as the mean of at least 3 richest hpf observed at nasal cytology according to our previous experience 13-17.
Statistical analyses
Statistical analysis was performed using SPSS 25 for Windows (IBM SPSS Statistics for Windows, Version 25.0. IBM Corp: Armonk, NY, USA). We created an electronic database to collect all study variables using Microsoft Excel, Microsoft Corporation (2018). All data were anonymised and shared between researchers without personal data. Continuous normally distributed data was expressed as mean ± standard deviation (SD) while non-normally distributed data was expressed as medians (interquartile range).
Continuous values, such as levels of blood eosinophils, symptom scores, and eosinophil count at nasal cytology were expressed as mean ± SD. Chi-square test was used to detect significant differences for qualitative variables, while correlations were assessed using logistic regression for linear and binary variables and Pearson’s correlation coefficient for correlations between continuous variables. The t-test for paired samples and the Kolmogorov-Smirnov test were used for normally distributed data. We used Wilcoxon Signed Rank test to analyze data that were not normally distributed. Statistical significance was assumed for p values < 0.05.
In order to evaluate the correlation between nasal cytology findings and clinical presentation, we analysed the VAS and SNOT scores based on 2 different established cut-offs for nasal eosinophilia, namely 5 cells/hpf 11 and 10 cells/hpf 3,7. To assess whether blood eosinophilia correlates with the severity of CRSwNP, we analysed VAS and SNOT scores based on 2 different established eosinophilia cut-offs (500 cells/mm3 and 1500 cells/mm3). Eosinophilia is commonly defined in the literature as an absolute eosinophil count (AEC) > 500 cells/mm3 and can be classified as mild (500-1500 cells/mm3), moderate (1500 to 5000 cells/mm3) or severe (> 5000 cells/mm3) 1-24.
Results
Clinical characteristics and phenotyping of patients are reported in Table I.
Prevalence of blood eosinophilia count in CRSwNP and in subpopulations
In this series, 390 of 425 patients enrolled (82.6%) had serum levels of eosinophils > 150 cells/mm3. Furthermore, we observed significantly higher mean levels of blood eosinophils in patients with CRSwNP and asthma compared to those with CRSwNP without asthma (910 ± 115 vs 550 ± 75, respectively, p < 0.01). We also observed significantly higher mean levels of blood eosinophils in patients with NSAID-ERD and asthma compared to those without these comorbidities (1220 ± 220 vs 550 ± 75) (p < 0.01). We found significantly higher mean levels of absolute eosinophil blood count in CRSwNP patients with documented allergy to skin prick test compared to those without allergy (950 ± 120 vs 570 ± 70, respectively, p < 0.05). We did not observe a significant difference in blood eosinophils in patients who underwent more than one functional endoscopic sinus surgery (FESS) compared to those who underwent ≤ 1 FESS (440 ± 65 vs 551 ± 70, p = N.S.).
Prevalence of nasal eosinophilia measured by nasal cytology in CRSwNP and in subpopulations
In this series, 274 of 425 patients (64%) presented eosinophilia at nasal cytology (> 1 eosinophil/hpf as a mean of at least the 3 richest fields). In the overall population, the mean eosinophil count at nasal cytology was 3.8 ± 1.2 cells/hpf. We observed that the mean eosinophil count at nasal cytology was significantly higher in patients with asthma and CRSwNP compared to those with CRSwNP without asthma (4.7 ± 1.3 vs 2.7 ± 1.2, respectively, p < 0.05). In CRSwNP patients with NSAID-ERD and asthma, mean levels of nasal eosinophilia, compared to those with non-comorbid CRSwNP, were significantly higher (6.4 ± 1.9 vs 3.0 ± 1.4, respectively, p < 0.05). We also found significantly higher mean levels of nasal eosinophil counts in CRSwNP patients with documented allergy to inhalants at skin prick-test compared to those without allergy (4.2 ± 1.5 vs 2.6 ± 1.2, respectively, p < 0.05). Furthermore, we analysed levels of nasal eosinophils based on number of previous sinonasal surgeries: we observed a higher eosinophil count in patients who had > 1 FESS compared to those who had ≤ 1 FESS (6.5 ± 1.8 versus 3.4 ± 1.4, respectively, p < 0.05). Finally, among the 151 patients without eosinophils at nasal cytology, 49 (32%) had serum eosinophils > 150 cells/mm3.
We did not observe a correlation between peripheral and local eosinophilia analysed by Spearman’s linear regression (R = 0.216).
Correlation between blood eosinophilia with quality of life and VAS symptom scores
Patients who had an absolute eosinophil blood count < 500 cells/mm3 had an average SNOT-22 value of 42 ± 14, while patients with an absolute eosinophil blood count ≥ 500 cells/mm3 had an average SNOT-22 value of 39.5 ± 14, although the difference was not statistically significant. Moreover, comparing median VAS scores for nasal obstruction, rhinorrhoea, smell impairment, and cranio-facial pain there were no significant differences between the 2 subpopulations identified with a cut-off of 500 cells/mm3 (Tab. II).
Patients who had an absolute eosinophil blood count <1500 cells/mm3 had an average SNOT-22 value of 40 ± 15, while those with an absolute eosinophil blood count ≥ 1500 cells/mm3 had an average SNOT-22 value of 39.5 ± 14 (p = N.S.). Similarly, comparing median VAS score for nasal obstruction, rhinorrhoea, smell impairment, and cranio-facial pain we did not observe significant differences between the 2 subpopulations with a cut-off of 1500 eosinophils/mm3 (Tab. II).
Finally, there was no significant correlation between mean absolute eosinophil blood count values and mean SNOT-22 score (p = 0.8, R = 0.13).
Correlation between local eosinophilia with quality of life and VAS symptom scores
On the other hand, we observed a different scenario when estimating SNOT-22 scores in different subgroups of patients identified based on levels of nasal eosinophils. Namely, we divided the study population into different subgroups considering 2 different cut-offs: 5 eosinophils/hpf and 10 eosinophils/hpf at nasal cytology.
Patients with eosinophil count ≥ 10 cells/hpf had a significantly higher median VAS for nasal obstruction, rhinorrhoea, smell impairment, and cranio-facial pain than patients with eosinophil count < 10.
We then repeated the analyses, lowering the cut-off to < 5 eosinophils/hpf at nasal cytology, and found comparable results. In particular, the mean values of all the parameters analysed were significantly higher in patients with eosinophil count ≥ 5 cells/hpf compared to those with eosinophil count < 5 cells/hpf (p < 0.05) as shown in Table III and Figures 1-2. Moreover, patients who had a local eosinophil count < 10 cells/hpf had a significantly lower mean SNOT-22 score compared to those with local eosinophil count ≥ 10 cells/hpf (39 ± 13 cells/hpf versus 49.5 ± 14 cells/hpf; p < 0.05). We repeated the analyses lowering the cut-off to 5 eosinophils/hpf at nasal cytology and found comparable results (Fig. 3; Tab. III).
Discussion
In Western countries, CRSwNP is mainly associated with eosinophil-dominant Type 2 inflammation involving various cytokines (e.g., IL-4, IL-5, IL-13, and IL-33) that regulate the proliferation and differentiation of eosinophils, thereby affecting their transmigration and enhanced survival in peripheral sinonasal mucosa. Eosinophilic inflammation has been extensively studied in sinonasal mucosa and has been associated with greater symptom severity, poorer disease control, and less response to medical and surgical treatment with higher recurrence rates of nasal polyps after surgery 14,18,19.
The search for biomarkers that can define the presence of Type 2 inflammation and severity of the disease is a trending topic 16,17,20,21 and has become increasingly important with the arrival of new biological drugs that target the pivotal Type 2 cytokines 22. Unfortunately, the clinical use of biomarkers in CRSwNP is very limited due to the conflicting data in the literature 22-24. Considering the limited availability of biomarkers in CRSwNP, the position papers/guidelines take into consideration blood and local eosinophilia, on which more data are available, especially for the definition of Type 2 inflammation associated with the disease. In this regard, the CRS EUFOREA/EPOS expert group recently 1,24 confirmed a cut-off of 10 eosinophils/hpf for local eosinophilia and lowered the cut-off for blood eosinophilia from 250 to 150 eosinophils/mm3. Despite the supporting evidence provided by guidelines, unfortunately clinicians do not routinely use these biomarkers as recently demonstrated in a national survey in Italy 23. For all these reasons, herein we investigated the prevalence of these biomarkers in CRSwNP and subpopulations and aimed to determine if they are indicative of disease severity, assessed in terms of impairment of quality of life and symptom.
From a clinical point of view, stratification of clinical severity may be very useful in routine practice to identify patients with a poor chance of achieving control with surgery, to modulate medical therapy, and to focus on potential candidates for personalised targeted therapy. Several studies have demonstrated that the presence of local eosinophilia is frequently associated with more severe disease, higher recurrence rates, and shorter disease-free intervals after treatment compared to non-eosinophilic forms. Histological evaluation of the eosinophilic inflammatory infiltrate on biopsy or on previous histopathological findings represents a preferred method to define mucosal eosinophilia, but remains burdened by the need for an invasive approach that is not easily repeatable on a routine basis. Some authors 18, to avoid the need for tissue biopsy, have demonstrated that nasal cytology may be used to study inflammatory pathways in CRS patients. Furthermore, nasal cytology is non-invasive and easy to perform with significant diagnostic and prognostic implications 6-8. In this regard, nasal cytology could represent a valid alternative to biopsy and can be performed routinely and repeatedly in clinical practice as in our previous experience 13-15, 25.
On the other hand, the role of absolute eosinophil blood count is increasing, especially because it may play an important role in the definition of Type 2 CRS. Nevertheless, circulating eosinophil counts can be falsely elevated by comorbid parasite infection, allergy, autoimmune disorders, or adverse events. Indeed, levels of absolute eosinophil blood count may be influenced by comorbidities and especially by asthma. Both asthma and CRSwNP share similar pathophysiological driving mechanisms underlying the disease, indicating mucosal sensitivity to chronic stimulus and being the main source of chronic stimulus 26. Past experiences in the literature confirm that eosinophilia may be present more frequently in CRSwNP patients with comorbid asthma compared to those with CRSwNP alone 26,27. Nevertheless, it is not clearly understood whether an asthma eosinophilic phenotype, according to a peripheral blood criterion, is related to severity of disease, lack of symptom control, or presence of airway obstruction.
In this study, 82.6% of CRSwNP patients had serum levels of eosinophils > 150 cells/mm3: this confirms that absolute eosinophil blood count may be clinically useful to define Type 2 inflammation 24. In our population, we observed that CRSwNP patients with comorbidities (asthma, NSAID-ERD, and allergies) were associated with higher levels of absolute eosinophil blood count compared to those without these conditions. For this reason, careful differential diagnosis of associated comorbidities is always recommended in case of high blood eosinophil values. On the other hand, the percentage of patients who had local eosinophilia at nasal cytology was somewhat lower (64%). Although methodological-related problems might have influenced this, we believe that eosinophil-positive cytology can also be a useful clinical biomarker to support the definition of Type 2 inflammation in patients with CRSwNP.
Our data confirm that blood eosinophil count does not correlate with nasal eosinophil count: this demonstrates that mucosal eosinophil count and blood eosinophils can vary greatly from patient to patient, showing distinct subgroups: patients with simultaneously increased blood and tissue eosinophil levels, patients with isolated tissue eosinophilia, and patients with isolated increased blood eosinophils. Of note, among 122 patients with no increase in blood eosinophils, 43 (35%) had a positive nasal cytology for eosinophilic infiltrate. Therefore, there is a subset of patients without increased blood eosinophils who test positive for eosinophils at nasal cytology. In cases like these, nasal cytology may be particularly useful in helping to define CRSwNP associated with Type 2 inflammation.
Regarding the relationship with symptom severity and quality of life, we confirmed that nasal eosinophil count may be associated with more severe symptoms and a greater burden on quality of life; on the other hand, this was not observed for blood eosinophils. In a recent research paper, Kowalik et al. demonstrated that blood eosinophil count correlated positively with clinical findings (Lund Mackay score and SNOT-22), although they enrolled patients with CRS in general 27. For this reason, their data are not comparable with ours because we enrolled only patients with CRSwNP. For local eosinophil count at nasal cytology, we subdivided patients based on 2 different cut-offs: we first performed the analysis using the cut-off of 10 cells/hpf suggested from guidelines for the definition of Type 2 inflammation 1. We detected significantly higher SNOT-22 and VAS scores for nasal obstruction, smell impairment, rhinorrhoea, and cranio-facial pain in patients with nasal eosinophil value greater than 10 cells/hpf. The same trend was maintained when lowering the cut-off to 5 eosinophils/hpf, which has also been used to define other diseases associated with Type 2 inflammation in the past 27. These certainly are preliminary data which, if confirmed, may be useful in identifying patients with greater severity of symptoms in real-life clinical practice. We repeated the same analysis using 2 different cut-offs for absolute eosinophil blood count: more specifically, we used the cut-off of 500 eosinophils/mm3 commonly used to define mild eosinophilia, and the cut-off of 1500 eosinophils/mm3 also used in the literature to define hyper-eosinophilia, and found no significant differences between subgroups. Finally, we correlated the levels of blood and local eosinophil counts with the number of previous surgeries, and noted that patients who had undergone more than one surgical procedure in the past had higher levels of local eosinophils, but not blood eosinophils. This additional finding would confirm that local eosinophilia is correlated to disease severity, while absolute eosinophil blood count is not.
Conclusions
Our results demonstrate that absolute eosinophil blood count and nasal cytology may represent useful tools in routine clinical practice to define Type 2 inflammation associated with CRSwNP. A higher mean blood and local eosinophilic count was observed in patients with comorbidities (asthma, allergic rhinitis, NSAID-ERD) than in those without these conditions. We did not observe a correlation between blood and local eosinophil counts. We demonstrated that local eosinophil count is correlated with greater severity of symptoms and higher SNOT-22 scores starting from a cut-off of > 5 eosinophils/hpf at nasal cytology. In addition, patients with more than one prior surgery showed higher levels of local eosinophils count. On the other hand, absolute eosinophils blood count was not correlated with severity of symptoms, SNOT-22 score, or number of previous surgeries.
Conflict of interest statement
The authors declare no conflict of interest.
Fundings
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
EDC: conception and design of the study, acquisition of the data, analysis, and interpretation of the data; drafted the article and revised it for important intellectual content; gave final approval of the version to be submitted; agree to be accountable for all aspects of the work. CM: drafted the article and revised it for important intellectual content; MC, SV, DCT, PC, BS, MR, DAG, DALM, PC: acquisition of the data, analysis and interpretation of the data; gave final approval of the version to be submitted; agree to be accountable for all aspects of the work. JG: final approval of the version to be submitted.
Ethical consideration
This study was approved by the Institutional Ethics Committee (Fondazione Policlinico Universitario Agostino Gemelli IRCCS). Protocol number: ID 36127/19 ID2758. The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki. Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: December 24, 2023
Accepted: August 8, 2024
Figures and tables
Figure 1. VAS nasal obstruction and smell impairment in patients with < 5 eosinophils/hpf or ≥ 5 eosinophils/hpf at nasal cytology.
Figure 2. VAS rhinorrhoea and cranio-facial pain in patients with < 5 eosinophils/hpf or ≥ 5 eosinophils/hpf at nasal cytology.
Figure 3. Mean SNOT-22 score subdividing patients based on nasal cytology eosinophil count assuming a cut-off of 5 eos/hpf or 10 eos/hpf.
| Age (mean ± SD; range) | 51.9 years ± 15; range 23-75 |
| Female (n/total; %) | 207/425; 48.7% |
| Male (n/total; %) | 218/425; 51.3 % |
| Concomitant allergic rhinitis (n/total; %) | 139/425; 33% |
| Concomitant asthma (n/total; %) | 139/425; 33% |
| NSAID-ERD (n/total; %) | 44/425; 10.3% |
| Family history for CRSwNP (n/total; %) | 114/425; 27% |
| Smoking (n/total; %) | 64/425; 15% |
| Peripheral blood eosinophils > 150 cells/mm3 (n/total; %) | 35/425; 82.6% |
| Eosinophils at nasal cytology (n/total; %) (> 1 eosinophil/hpf as a mean of at least the most 3 richest fields) | 274/425; 64% |
| Previous sinonasal surgery (n/total; %) | 303/425; 71% |
| Number of previous sinonasal surgeries (mean ± SD | 1.7 ± 1 |
| SNOT-22 score (mean ± SD) | 39.2 ± 17.6 |
| VAS nasal obstruction (mean ± SD) | 6.7 ± 2.5 |
| VAS smell (mean ± SD) | 5.3 ± 3.7 |
| VAS rhinorrhoea (mean ± SD) | 5.7 ± 3.1 |
| VAS cranio-facial pain (mean ± SD) | 4.1 ± 2.1 |
| CT Lund Mackay score (mean ± SD) | 13 ± 6.4 |
| Absolute eosinophil blood count | |||
|---|---|---|---|
| < 500 cells/mm 3 | ≥ 500 cells/mm 3 | P | |
| VAS nasal obstruction ° | 8(2) | 8(1) | N.S. |
| VAS rhinorrhoea ° | 8(2) | 8(2) | N.S. |
| VAS smell ° | 7(3) | 8(3) | N.S. |
| VAS cranio-facial pain ° | 3(1) | 4(2) | N.S. |
| SNOT-22 * | 42 ± 14 | 39.5 ± 14 | N.S. |
| < 1500 cells/mm 3 | ≥ 1500 cells/mm 3 | P | |
| VAS nasal obstruction ° | 8(2) | 8(2) | N.S. |
| VAS rhinorrhoea ° | 8(2) | 8(2) | N.S. |
| VAS Smell ° | 8(3) | 7(2) | N.S. |
| VAS cranio-facial pain ° | 3(1) | 4(2) | N.S. |
| SNOT-22 * | 40 ± 15 | 39.5 ± 14 | N.S. |
| VAS: visual analogue scale; SNOT: sinonasal outcome test; N.S.: non-significant; *Continuous data are expressed as mean ± SD; °non-normally distributed data are expressed as median (interquartile range). | |||
| Eosinophil count at nasal cytology | |||
|---|---|---|---|
| < 5 eosinophils/hpf | ≥ 5 eosinophils/hpf | P | |
| VAS nasal obstruction ° | 7(3) | 8(1) | p < 0.01 |
| VAS rhinorrhoea ° | 7(5) | 8(2) | p < 0.05 |
| VAS smell ° | 5(8) | 9(2) | p < 0.01 |
| VAS cranio-facial pain ° | 2(3) | 4(5) | p < 0.05 |
| SNOT-22 * | 37.9 ± 12.6 | 51.1 ± 11.1 | p < 0.0001 |
| < 10 eosinophils/hpf | ≥ 10 eosinophils/hpf | P | |
| VAS nasal obstruction ° | 7(3) | 8(1) | p < 0.01 |
| VAS rhinorrhoea ° | 7(5) | 8(2) | p < 0.05 |
| VAS smell ° | 5(8) | 9(2) | p < 0.01 |
| VAS cranio-facial pain ° | 3(4) | 5(5) | p < 0.01 |
| SNOT-22 * | 39 ± 13.5 | 49.5 ± 10.4 | p < 0.01 |
| VAS: visual analogue scale; SNOT: sinonasal outcome test; *Continuous data are expressed as mean ± SD; °non-normally distributed data are expressed as median (interquartile range). | |||
References
- Fokkens W, Lund V, Hopkins C. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58:1-464. doi:https://doi.org/10.4193/Rhin20.600
- De Corso E, Bilò B, Matucci A. Personalized management of patients with chronic rhinosinusitis with nasal polyps in clinical practice: a multidisciplinary consensus statement. J Pers Med. 2022;12. doi:https://doi.org/10.3390/jpm12050846
- Ho J, Hamizan A, Alvarado R. Systemic predictors of eosinophilic chronic rhinosinusitis. Am J Rhinol Allergy. 2018;32:252-257. doi:https://doi.org/10.1097/ACI.0000000000000602
- Aslan F, Altun E, Paksoy S. Could eosinophilia predict clinical severity in nasal polyps?. Multidiscip Respir Med. 2017;12. doi:https://doi.org/10.1186/s40248-017-0102-7
- Nakayama T, Yoshikawa M, Asaka D. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology. 2011;49:392-396. doi:https://doi.org/10.4193/Rhino10.261
- Bhattacharyya N, Vyas D, Fechner F. Tissue eosinophilia in chronic sinusitis: quantification techniques. Arch Otolaryngol Head Neck Surg. 2001;127:1102-1105. doi:https://doi.org/10.1001/archotol.127.9.1102
- De Corso E, Seccia V, Ottaviano G. Clinical evidence of type 2 inflammation in non-allergic rhinitis with eosinophilia syndrome: a systematic review. Curr Allergy Asthma Rep. 2022;22:29-42. doi:https://doi.org/10.1007/s11882-022-01027-0
- De Corso E, Battista M, Pandolfini M. Role of inflammation in non-allergic rhinitis. Rhinology. 2014;52:142-149. doi:https://doi.org/10.4193/Rhino13.102
- Soler Z, Sauer D, Mace J. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010;142:64-71. doi:https://doi.org/10.1016/j.otohns.2009.10.005
- Gallo S, Bandi F, Preti A. Exploring the role of nasal cytology in chronic rhinosinusitis. Acta Otorhinolaryngol Ital. 2020;40:368-376. doi:https://doi.org/10.14639/0392-100X-N0711
- Chowdhury N, Mace J, Bodner T. Investigating the minimal clinically important difference for SNOT-22 symptom domains in surgically managed chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7:1149-1155. doi:https://doi.org/10.1002/alr.22028
- Toma S, Hopkins C. Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhinology. 2016;54:129-133. doi:https://doi.org/10.4193/Rhino15.07
- De Corso E, Baroni S, Settimi S. Sinonasal biomarkers defining type 2-high and type 2-low inflammation in chronic rhinosinusitis with nasal polyps. J Pers Med. 2022;12. doi:https://doi.org/10.3390/jpm12081251
- De Corso E, Settimi S, Tricarico L. Predictors of disease control after endoscopic sinus surgery plus long-term local corticosteroids in CRSwNP. Am J Rhinol Allergy. 2021;35:77-85. doi:https://doi.org/10.1177/1945892420936196
- De Corso E, Lucidi D, Battista M. Prognostic value of nasal cytology and clinical factors in nasal polyps development in patients at risk: can the beginning predict the end?. Int Forum Allergy Rhinol. 2017;7:861-867. doi:https://doi.org/10.1002/alr.21979
- De Corso E, Baroni S, Battista M. Nasal fluid release of eotaxin-3 and eotaxin-2 in persistent sinonasal eosinophilic inflammation. Int Forum Allergy Rhinol. 2014;4:617-624. doi:https://doi.org/10.1002/alr.21348
- De Corso E, Battista M, Pandolfini M. Role of inflammation in non-allergic rhinitis. Rhinology. 2014;52:142-149. doi:https://doi.org/10.4193/Rhino13.102
- Gelardi M, Iannuzzi L, De Giosa M. Non-surgical management of chronic rhinosinusitis with nasal polyps based on clinical-cytological grading: a precision medicine-based approach. Acta Otorhinolaryngol Ital. 2017;37:38-45. doi:https://doi.org/10.14639/0392-100X-1417
- Pan L, Liao B, Guo C. Inflammatory features and predictors for postsurgical outcomes in patients with nasal polyps stratified by local and systemic eosinophilia. Int Forum Allergy Rhinol. 2021;11:846-856. doi:https://doi.org/10.1002/alr.22702
- De Corso E, Baroni S, Lucidi D. Nasal lavage levels of granulocyte-macrophage colony-stimulating factor and chronic nasal hypereosinophilia. Int Forum Allergy Rhinol. 2015;5:557-562. doi:https://doi.org/10.1002/alr.21519
- De Corso E, Baroni S, Romitelli F. Nasal lavage CCL24 levels correlate with eosinophils trafficking and symptoms in chronic sino-nasal eosinophilic inflammation. Rhinology. 2011;49. doi:https://doi.org/10.4193/Rhino10.133
- De Corso E, Bellocchi G, De Benedetto M. Biologics for severe uncontrolled chronic rhinosinusitis with nasal polyps: a change management approach. Consensus of the Joint Committee of Italian Society of Otorhinolaryngology on biologics in rhinology. Acta Otorhinolaryngol Ital. 2022;42:1-16. doi:https://doi.org/10.14639/0392-100X-N1614
- De Corso E, Pipolo C, Cantone E. Practical recommendations for managing severe chronic rhinosinusitis with nasal polyps in the era of biologics. Acta Otorhinolaryngol Ital. 2023;43:324-340. doi:https://doi.org/10.14639/0392-100X-N2422
- Fokkens W, Viskens A, Backer V. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology. 2023;61:194-202. doi:https://doi.org/10.4193/Rhin22.489
- De Corso E, Anzivino R, Galli J. Antileukotrienes improve naso-ocular symptoms and biomarkers in patients with NARES and asthma. Laryngoscope. 2019;129:551-557. doi:https://doi.org/10.1002/lary.27576
- Li F, Wang X, Shen S. Risk factors associated with comorbid asthma in patients with chronic rhinosinusitis with nasal polyps: a cross-sectional study. BMC Pulm Med. 2022;22. doi:https://doi.org/10.1186/s12890-022-02138-0
- Kowalik K, Waniewska-Leczycka M, Sarnowska E. The SWI/ SNF complex in eosinophilic and non eosinophilic chronic rhinosinusitis. Acta Otorhinolaryngol Ital. 2021;41:159-167. doi:https://doi.org/10.14639/0392-100X-N0760
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 558 times
- PDF downloaded - 270 times




 PDF
PDF
