Laryngology
Vol. 45: Issue 1 - February 2025
Bama pig is a suitable animal for studying laryngopharyngeal reflux disease
Abstract
Objective. To assess the suitability of Bama pigs as a model for laryngopharyngeal reflux disease (LPRD) research.
Methods. Sixteen 8-month-old male Bama pigs underwent esophageal manometry to determine the precise anatomical positioning of the upper (UES) and lower esophageal sphincters (LES) relative to the incisor teeth, as well as their respective contraction intensities. The pigs were randomly allocated into three experimental groups (n = 6, 5, 5), with each group subjected to Dx PH-probe monitoring. In Group 1, animals were fasted for 24 hours and water-deprived for 6 hours before undergoing pH monitoring under anaesthesia. Group 2 was anaesthetised two hours post-normal feeding and subsequently monitored. Group 3 also received anaesthesia two hours after eating but were monitored in an awake state.
Results. The mean distance from the UES to the incisor teeth was found to be 19.8 ± 1 cm, while the LES was located at 40 ± 2.5 cm. The resting pressure measurements revealed a mean value of 64 ± 12 mmHg for the UES and 20 ± 4 mmHg for the LES in Bama pigs. Laryngopharyngeal pH values across the three groups were 7 ± 0.6, 7 ± 0.5, and 7.4 ± 1.2, respectively, showing no significant differences or reflux events. Similarly, there was no statistically significant difference in the lower oesophageal pH between Group 1 and Group 2.
Conclusions. The Bama pig emerges as a suitable animal model for studying LPRD, given its comparable physiological parameters. The feasibility of establishing a reflux model in Bama pigs and using it to investigate the underlying mechanisms of LPRD is convincingly supported by these findings.
Introduction
Laryngopharyngeal reflux disease (LPRD) constitutes a pathological condition characterised by the retrograde movement of gastric contents beyond the upper esophageal sphincter, thereby manifesting in a constellation of symptoms and signs 1. Globally pervasive, LPRD represents a significant health concern with documented prevalence rates ranging from 9.3% in Italy 2 to 10.1% in China 3, escalating to 11% in India 4 and 18.8% in Greece 5, and reaching as high as 34.4% in the United Kingdom 6, thus illustrating its extensive occurrence across various geographic areas and populations. Similarly, as another condition related to reflux, gastro-esophageal reflux disease (GERD) also exhibits a considerable prevalence, affecting, for instance, up to 38.5% of the population in Italy, thereby highlighting its significant health burden 2. Initially recognised as an extra-esophageal manifestation of GERD, LPRD has exhibited strong associations with GERD. Notably, 71% of such individuals experienced hoarseness while 51% reported persistent cough, indicating a potential correlation between GERD and LPRD 7. However, through deeper investigation, a consensus among experts emerged that although a connection exists, there are differences between these two diseases 8. While both conditions relate to the reflux of stomach contents, it is noteworthy that not all patients diagnosed with LPRD report typical acid reflux or heartburn symptoms, in contrast to those suffering from GERD 9. Given the substantial incidence and medical burden on affected individuals, it is imperative to delve into the underlying mechanisms of LPRD in order to enhance diagnostic precision and therapeutic strategies.
The pathophysiological intricacies of LPRD have been the subject of extensive research over several years, largely through the development and utilisation of diverse animal models. Among these experimental animals, which typically include mice, rats, guinea pigs, rabbits, and pigs, the pig has emerged as a more favourable option for LPRD studies. The porcine larynx is known to exhibit structural similarities with that of humans 10, rendering it a suitable proxy for investigating analogous pathological processes. When exposed to gastric refluxate, pigs display pertinent pathological alterations in their throat mucosa, such as inflammation, widened intercellular spaces, and diminished desmosomes 11, thereby providing valuable insights into the fundamental mechanisms underlying LPRD. Previous researchers have indeed constructed Bama pig models of laryngopharyngeal reflux, yet these endeavours did not focus on the physiological attributes specific to this species 11. In this context, our study uniquely presents an in-depth characterisation of the upper aerodigestive tract properties in Bama pigs and critically discusses the rationale for inducing laryngopharyngeal reflux.
Given the fact that laryngopharyngeal reflux arises from the daily occurrence of gastric contents reflux and in light of the influence of anaesthesia medications and dietary intake on gastrointestinal motility, this study uniquely examines the pH value characteristics within the laryngopharynx of healthy Bama pigs under a spectrum of distinct conditions. Specifically, we explore the differences in pH values between anaesthetised and awake states, as well as during fasting compared to non-fasting intervals. This meticulous investigation into the effects of these variables represents a novel and innovative facet of our research endeavour.
Materials and methods
Animals
Sixteen male Bama pigs were procured from the Beijing Shichuang Century Minipig Breeding Facility. These 8-month-old animals exhibited an average length of 75.5 ± 2 cm and weighed 13.2 ± 1.8 kg each. Prior to commencing experimentation, the pigs were acclimatised for a week in an environment with optimised temperature and humidity conditions, and they received a twice-daily feeding regimen to ensure adequate adaptation. Subsequently, these 16 subjects were randomly assigned into three experimental groups (n = 6, 5, 5), respectively, for pH monitoring under three distinct sets of circumstances. It is noteworthy that all experimental protocols were sanctioned by the Institutional Animal Care and Use Committee (IACUC) of our hospital, thereby ensuring compliance with ethical standards throughout the study process.
Esophageal manometry
A four-channel esophageal manometry system (MMS, Netherlands) was employed to determine the spatial relationship between the upper and lower esophageal sphincters (UES and LES), relative to the incisor teeth, as well as to measure their respective resting pressures. Following calibration, the manometric catheter was orally inserted into the Bama pig’s esophagus under endoscopic guidance to ensure accurate placement. Upon gradual advancement of the device, two discernible pressure peaks were identified, which corresponded to the resting pressures at the UES and LES. Concurrently, the precise distance between these two sphincteric structures and the incisor teeth could be directly read from the scale on the equipment. The resting pressure for each sphincter was defined as the mean value obtained from a 10-minute continuous recording period, thus providing a reliable representation of their baseline tonic state.
Ambulatory pH monitoring
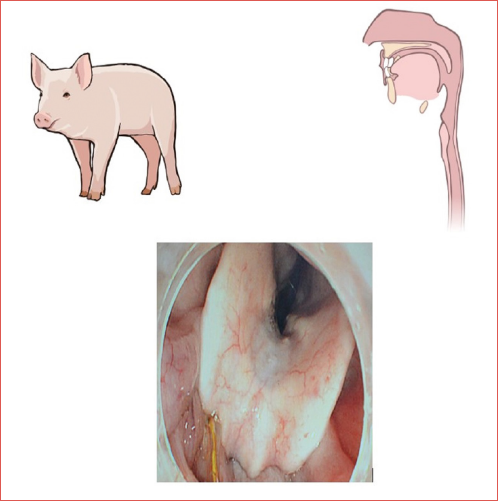
The pH monitoring process was executed utilising the Dx-pH system (Respiratory Technology Corporation, San Diego, CA), which was selected for its technical specifications and suitability for this investigative endeavour. In accordance with the predefined experimental stratification, distinct pre-procedural treatments were administered to each group. Among these, Bama pigs within the first experimental cohort (n = 6) underwent a regimen of fasting from solid food for a period of 24 hours, accompanied by abstinence from water consumption for the final 6 hours preceding the commencement of the experimental protocol. Anaesthesia was conducted by intramuscular injection of 1 mg ketamine and tracheal intubation was used to maintain sufficient oxygen supply. Lumina was given 1 mg intermittently to maintain the depth of anaesthesia and vital signs of Bama pigs were continuously evaluated to prevent accidents. Following meticulous calibration in buffer solutions at pH 4 and 7, a Dx-pH probe was positioned at the level of the esophageal entrance proximal to UES for the purpose of 2h laryngopharyngeal pH monitoring (Fig. 1). Concurrently, another probe was placed precisely 3 cm above LES, guided by the outcomes of esophageal manometry, and maintained there for a continuous period of two hours (Fig. 2). The second experimental group, comprising five subjects, underwent feeding 2h prior to the commencement of pH monitoring and thereafter followed an identical protocol involving anaesthesia administration, probe placement procedures, and a subsequent two-hour pH monitoring session. In contrast, the third group, also consisting of five subjects, mirrored the preoperative preparation regimen of the second group; however, a key divergence lay in the cessation of anaesthesia following the secure fixation of the electrode. The pH monitoring process was initiated and sustained for a duration of two hours immediately upon the attainment of full consciousness by the Bama pigs. Due to the inherent restriction imposed by the abbreviated length of the Dx-pH catheter, measuring 46 cm, it proved challenging to ensure a proper fixation of the probe in the lower esophageal region of an awake Bama pig (Fig. 3). Consequently, for the subjects in the third experimental group, pH monitoring was confined solely to the laryngopharyngeal region. The entirety of the collected monitoring data was processed using Dataview Lite V3 software, and from these readings, an average pH value was computed accordingly.
Statistical analysis
SPSS 27.0 statistics software was used to analyse data. Data was presented as x ± s if obeying normal distribution, and described by median (interquartile range) if not. The non-parametric tests were used to determine whether significant difference existed among different groups’ pH monitoring results.
Results
Distance of UES and LES from incisor teeth
The results of esophagus manometry of 16 Bama pigs revealed that UES was located at the distance of 19.8 ± 1 cm from the incisor teeth, while LES was 40 ± 2.5 cm from the incisors.
Resting pressure of UES and LES
The average resting pressure of UES and LES was 64 ± 12 mmHg and 20 ± 4 mmHg, respectively. The contractile force of UES was significantly stronger than LES, suggesting that its impact on the development of LPRD cannot be ignored.
Dx-pH monitoring results
No reflux events were observed in all laryngopharyngeal pH monitoring processes. The laryngopharyngeal pH of three groups was 7 ± 0.6, 7 ± 0.5, and 7.4 ± 1.2, respectively. There was no significant difference among three groups’ results (Tab. I, p > 0.05). The pH of Bama pigs’ lower esophagus of the first and second group were 6.2 ± 1 and 7 ± 0.8, respectively, showing no significant difference (Tab. II, p > 0.05).
Discussion
LPRD was initially postulated by Koufman in 1991 7 and later gained recognition within the American Academy of Otolaryngology - Head and Neck Surgery in 2002 12. Initially, LPRD was conceptualised as an extra-esophageal manifestation of GERD, yet its understanding was impeded due to a lack of clear definitional boundaries. A pioneering study involving GERD patients revealed that nearly one-third exhibited laryngopharyngeal symptoms, such as chronic cough 13. With the advent of more extensive research, GERD has been precisely defined as a condition arising from the retrograde movement of gastric contents with a pH < 4 across LES into the esophagus. In contrast, LPRD is characterised by the reflux of stomach contents beyond UES, which can be categorised into acid and non-acid reflux types 14. Notably, the laryngeal mucosa exhibits heightened susceptibility to reflux-induced damage compared to the esophageal mucosa 15; thus, less frequent and higher pH reflux events are sufficient to trigger LPRD. Empirical evidence suggests that the esophagus can tolerate up to 50 reflux episodes per day without significant injury, whereas reflux events occurring merely three times weekly may be considered pathological for laryngopharyngeal tissues 14, highlighting the differential tolerance threshold between these anatomical regions.
The abnormality of structure or function of anti-reflux barriers plays a key role in the development of LPRD, especially the decrease in contractility of UES and LES 16. According to a survey conducted in people free from LPRD and GERD, the normal pressure of UES is 56.16 ± 5.02 mmHg and of LES is 22.73 ± 5.53 mmHg 17. Clinically, Reflux Symptom Index (RSI) and Reflux Finding Score (RFS) formulated by Belafsky are common and convenient diagnostic approaches for LPRD but lack specificity 18, since some symptoms and signs can overlap with other diseases. Utilising the Reflux Symptom Score (RSS) encompassing a broader spectrum of symptoms proves to be a more reliable method for assessing the likelihood of LPRD in patients 19; however, a definitive diagnosis of LPRD cannot be solely based on subjective symptomatology alone. As a supplement, 24h multichannel intracavity impedance pH monitoring (24h MII-pH monitoring) is thought as the golden standard to make a diagnosis of LPRD 20. The mean laryngopharyngeal pH value is 7 and acidic reflux events are seldom detected in healthy volunteers undergoing 24h MII-pH monitoring 21. A reflux event with pH < 5.5 in the upright position or < 5 in the supine position caught by 24h MII-pH monitoring is considered abnormal. Dx-pH monitoring is also an objective method, which defines the diagnostic criteria as Ryan index > 9.41 in the upright position and/or > 6.79 in the supine position 1.
It is essential to figure out the pathogenesis of LPRD by establishing suitable animal models because of its complexity and uncertainty. LPR can be induced by endogenous and exogenous methods in animals, in which endogenous methods are more credible by mimicking its pathophysiological process 22. Mice, rats, guinea pigs, rabbits and pigs are all considerable choices to be used to conduct relevant studies. Marinho et al. found that inflammation of laryngeal tissue could be induced by inserting a nasogastric tube into rat’s stomach 23. By ligation of pylorus and gastric fundus, histological inflammatory changes were verified in rats’ upper aerodigestive mucosa by Shimazu et al. 24, while Habesoglu found that soft palate tissue in rats suffered from similar damage caused by reflux contents 25. Hu et al. successfully established LPR model by performing total cardiomyectomy in New Zealand rabbits and observed obvious submucous gland hyperplasia in the surgical group 26. Cao et al. also chose New Zealand rabbits to establish LPRD animal model and revealed that pepsin concentration in vocal cord increased significantly compared with a control group, which was induced by the simultaneous dilation of UES and LES 27.
Stimulated by low pH and pepsin, squamous epithelial proteins Sep70 and Sep53 were found to be significantly decreased in a porcine organ culture model in vitro, which was consistent with changes of LPRD patients’ laryngeal Sep70 and Sep53 28. Different parts of porcine laryngeal mucosa tended to response to acid and pepsin differently, mirroring changes caused by LPR in human larynx. In porcine, subglottic mucosa was most sensitive to damage of acid and pepsin, while posterior commissure was least 29. Considering the similarity of pig and human laryngeal mucosa, which both consisted of stratified squamous and respiratory-type epithelium 10, Feng et al. planted metal stents to dilate UES and LES and demonstrated the increased intercellular space and decreased desmosomes of laryngopharyngeal mucosa, indicating the probable mechanism with which reflux contents damaged the intercellular barriers 11.
Previous animal studies suggest that artificially induced relaxation of LES with or without UES can lead to the development of LPRD. Emphasising the important effect of esophageal sphincter on anti-reflux, it is essential to study underlying mechanisms and develop effective therapy of LPRD by animal models. There are a variety of animals to choose from, but not all of them can convincingly show the development of disease similar to the human body. Our research makes an understanding of characteristics of 8-month-old Bama pigs. Like humans 17, Bama pigs have significantly higher UES pressure than LES (64 ± 12 mmHg vs 20 ± 4 mmHg). Considering the effect of anaesthesia and diet on laryngopharyngeal pH, 16 Bama pigs have been divided into three groups and monitored. Among three groups’ monitoring results, the laryngopharyngeal pH was nearly 7 and not affected by diet and anaesthesia. Similarly, another research concluded that fasting or not had little effect on the incidence of LPR in people 30. Compared with healthy people, Bama pig has similar esophageal pressure gradient distribution and stable and neutral laryngopharyngeal pH. In histological examination of animals’ normal larynx, pigs and rabbits have stratified squamous and respiratory epithelium, rather than rat and mouse 10. Investigated by electron microscopy, the larynx mucosa in porcine presents a transition from stratified squamous epithelium in supraglottic region to pseudostratified squamous epithelium in subglottic region, which is different from what observed in the rabbit larynx 8. The physiological characteristics of pig larynx are the basis of its application to study LPRD. Moreover, the changes of tight junction molecule and E-cadherin expression in pig larynx caused by reflux contents is also comparable with human 10.
Compared with exogenous methods, LPRD induced by endogenous methods is more able to simulate pathological conditions and requires experimental animals’ high tolerance to surgery. Therefore, a relatively large species is a better option, like pig. Feng et al. established a Bama pig model for LPRD by stent implantation with 80% success rate. In view of the variability of stent position and the uncertainty of expansion effect, a new and feasible surgical procedure for LPRD model is worth considering. It is of great importance to reproduce the disease process in experiment when digging into the pathogenesis of LPRD. Previous research show that in pig’s larynx, intercellular connection can be damaged and expression of some proteins such as Sep70 and Sep53 can be diminished as a result of LPR, which is also proven in human. Different effects of reflux contents on pig larynx mucosa depend on different anatomical sites, which also mimics clinical changes in true LPRD. It is credible that new pathological changes observed in pig LPRD model can reflect relevant mechanisms in patients, which remains to be confirmed. Finally, as classic laboratory animal, pig can be raised in a standardised manner, and the price is moderate. Pigs of different ages, genders and weights can be chosen at will and are easily available.
Through the present study, we understood characteristics of the esophageal sphincter pressure and laryngopharyngeal pH value of Bama pigs. The 8-month-old Bama pig has a moderate size and can be easily obtained from breeding base. Modeling operation can be performed in Bama pigs with high tolerance to surgery to observe subsequent pathophysiological changes, which are expected to be verified in LPRD patients in the future. Considering these features, Bama pig is a suitable animal for studying LPRD.
Conclusions
LPRD is a common burden to patients visiting Otorhinolaryngology - Head and Neck Surgery clinics. People with LPRD always experience chronic symptoms which lower their quality of life. It is meaningful to understand the underlying mechanisms and carry out effective managements of LPRD clinically. It is reported that pig presents similar physiological larynx manifestations and pathological changes as humans when experiencing laryngopharyngeal reflux. Abnormal findings observed in pigs concerning LPRD can be considered for validation in patients. In our research, 8-month-old Bama pigs are characterised by higher UES pressure than LES and stable laryngopharyngeal pH of around 7 and can be considered a good experimental model.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research is supported by Beijing Natural Science Foundation (No .7222196).
Author contributions
QS, YW: contribute to the design and drafting of the work and approve the version to be published; LZ, HA: are responsible for the conception and revision of the work and approve its publication. All authors agree to be accountable for all aspects of the work.
Ethical consideration
All research procedures were approved by the Animal Care Committee of Peking University People’s Hospital (No. 2022PHE026).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
History
Received: August 26, 2023
Accepted: November 23, 2024
Figures and tables
Figure 1. Laryngopharyngeal pH monitoring. The Dx-pH probe is placed at the orifice of the esophagus above the UES to monitor laryngopharyngeal pH.
Figure 2. Lower esophagus pH monitoring. The Dx-pH probe is placed 3 cm above the LES to monitor pH value of lower esophagus.
Figure 3. Dx-pH probe fixation method of the third group. The probe located at the level of esophagus entrance is placed transnasally and sutured onto the skin of cheek (shown by the black arrow).
| Subgroup | Median (interquartile range) | H | p |
|---|---|---|---|
| First group | 7 (0.6) | 0.971 | 0.616 |
| Second group | 7 (0.5) | ||
| Third group | 7.4 (1.2) |
| Subgroup | Median (interquartile range) | Z | p |
|---|---|---|---|
| First group | 6.2(1) | -2.008 | 0.052 |
| Second group | 7 (0.8) |
References
- Experts consensus on diagnosis and treatment of laryngopharyngeal reflux disease. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51:324-326. doi:https://doi.org/10.3760/cma.j.issn.1673-0860.2016.05.002
- Mozzanica F, Robotti C, Ambrogi F. Gastro-esophageal reflux, laryngo-pharyngeal reflux symptoms, and dysphonia in the Italian population of the area of Milan: results of an internet survey. Minerva Gastroenterol. 2024;70:472-474. doi:https://doi.org/10.23736/s2724-5985.23.03380-6
- Xiao S, Li J, Zheng H. An epidemiological survey of laryngopharyngeal reflux disease at the otorhinolaryngology-head and neck surgery clinics in China. Eur Arch Otorhinolaryngol. 2020;277:2829-2838. doi:https://doi.org/10.1007/s00405-020-06045-0
- Mishra P, Agrawal D, Chauhan K. Prevalence of laryngopharyngeal reflux disease in Indian population. Indian J Otolaryngol Head Neck Surg. 2022;74:1877-1881. doi:https://doi.org/10.1007/s12070-020-01882-1
- Spantideas N, Drosou E, Bougea A. Laryngopharyngeal reflux disease in the Greek general population, prevalence and risk factors. BMC Ear Nose Throat Disord. 2015;15. doi:https://doi.org/10.1186/s12901-015-0020-2
- Kamani T, Penney S, Mitra I. The prevalence of laryngopharyngeal reflux in the English population. Eur Arch Otorhinolaryngol. 2012;269:2219-2225. doi:https://doi.org/10.1007/s00405-012-2028-1
- Koufman J. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1-78. doi:https://doi.org/10.1002/lary.1991.101.s53.1
- Koufman J. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J. 2002;81:7-9.
- Yılmaz T, Bajin M, Günaydın R. Laryngopharyngeal reflux and Helicobacter pylori. World J Gastroenterol. 2014;20:8964-8970. doi:https://doi.org/10.3748/wjg.v20.i27.8964
- Gill G, Buda A, Moorghen M. Characterisation of adherens and tight junctional molecules in normal animal larynx; determining a suitable model for studying molecular abnormalities in human laryngopharyngeal reflux. J Clin Pathol. 2005;58:1265-1270. doi:https://doi.org/10.1136/jcp.2004.016972
- Feng G, Zhang Z, Diao C. A bama minipig model of laryngopharyngeal reflux and the change of laryngopharyngeal mucosal ultrastructure. J Neurogastroenterol Motil. 2015;21:182-188. doi:https://doi.org/10.5056/jnm14113
- Koufman J, Aviv J, Casiano R. Laryngopharyngeal reflux: position statement of the Committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35. doi:https://doi.org/10.1067/mhn.2002.125760
- Jaspersen D, Kulig M, Labenz J. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD Study. Aliment Pharmacol Ther. 2003;17:1515-1520. doi:https://doi.org/10.1046/j.1365-2036.2003.01606.x
- Koufman J, Amin M, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg. 2000;123:385-388. doi:https://doi.org/10.1067/mhn.2000.109935
- Calvo-Henríquez C, Ruano-Ravina A, Vaamonde P. Is pepsin a reliable marker of laryngopharyngeal reflux? A systematic review. Otolaryngol Head Neck Surg. 2017;157:385-391. doi:https://doi.org/10.1177/0194599817709430
- Brown J, Shermetaro C. Treasure Island (FL) Ineligible Companies. StatPearls Publishing; 2023.
- Xiaoyong L. Esophageal Dynamics and Reflux Characteristics of Gastroesophageal Reflux Disease Complicated With Laryngopharyngeal Reflux Disease. Xinjiang Medical University; 2019.
- Kelchner L, Horne J, Lee L. Reliability of speech-language pathologist and otolaryngologist ratings of laryngeal signs of reflux in an asymptomatic population using the reflux finding score. J Voice. 2007;21:92-100. doi:https://doi.org/10.1016/j.jvoice.2005.09.004
- Lechien J, Bobin F, Muls V. Validity and reliability of the reflux symptom score. Laryngoscope. 2020;130:E98-E107. doi:https://doi.org/10.1002/lary.28017
- Pizzorni N, Ambrogi F, Eplite A. Magnesium alginate versus proton pump inhibitors for the treatment of laryngopharyngeal reflux: a non-inferiority randomized controlled trial. Eur Arch Otorhinolaryngol. 2022;279:2533-2542. doi:https://doi.org/10.1007/s00405-021-07219-0
- Meixiang C. Normal Values of 24-Hour Multichannel Intraluminal Impedance in Combination With PH Monitoring for the Detection of Laryngopharyngeal Reflux and the Study of Its Affecting Factors. Fujian Medical University; 2018.
- Asaoka D, Nagahara A, Matsumoto K. Current perspectives on reflux laryngitis. Clin J Gastroenterol. 2014;7:471-475. doi:https://doi.org/10.1007/s12328-014-0535-x
- Marinho R, Matos R, Santos J. Potential anti-inflammatory effect of low-level laser therapy on the experimental reflux laryngitis: a preliminary study. Lasers Med Sci. 2014;29:239-243. doi:https://doi.org/10.1007/s10103-013-1323-4
- Shimazu R, Kusano K, Kuratomi Y. Histological changes of the pharynx and larynx in rats with chronic acid reflux esophagitis. Acta Otolaryngol. 2009;129:886-892. doi:https://doi.org/10.1080/00016480802468161
- Habesoglu T, Habesoglu M, Sürmeli M. Histological changes of rat soft palate with exposure to experimental laryngopharyngeal reflux. Auris Nasus Larynx. 2010;37:730-736. doi:https://doi.org/10.1016/j.anl.2010.03.009
- Hu Y, Xu X, Chen S. Laryngoscopy findings and histological results in a rabbit gastroesophageal reflux model. Eur Arch Otorhinolaryngol. 2012;269:1939-1944. doi:https://doi.org/10.1007/s00405-012-1968-9
- Jie C, Lihong Z, Wenlun W. Establishment and research of a New Zealand rabbit model of laryngopharyngeal reflux. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;54:912-918. doi:https://doi.org/10.3760/cma.j.issn.1673-0860.2019.12.006
- Johnston N, Dettmar P, Lively M. Effect of pepsin on laryngeal stress protein (Sep70, Sep53, and Hsp70) response: role in laryngopharyngeal reflux disease. Ann Otol Rhinol Laryngol. 2006;115:47-58. doi:https://doi.org/10.1177/000348940611500108
- Bulmer D, Ali M, Brownlee I. Laryngeal mucosa: its susceptibility to damage by acid and pepsin. Laryngoscope. 2010;120:777-782. doi:https://doi.org/10.1002/lary.20665
- Hamdan A, Nassar J, Dowli A. Effect of fasting on laryngopharyngeal reflux disease in male subjects. Eur Arch Otorhinolaryngol. 2012;269:2361-2366. doi:https://doi.org/10.1007/s00405-012-2038-z
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 229 times
- PDF downloaded - 71 times




 PDF
PDF
